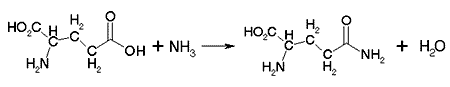
There are two major problems with this reaction proceding in the cell:
Lectures 4 and 5- Coupled Reactions and Molecular Recognition 9-2-98
Reading for today's class: pp 94-106
Reading for next class: pp 133-154
Consider the following reaction: glutamic acid + ammonia -> glutamine + H2O
Link to Dr. Minden's lecture notes.
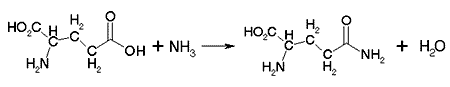
There are two major problems with this reaction proceding in the cell:
In the aqueous (or watery) environment of the all the reaction will gain or lose protons, which will make them changed and inhibit the reaction.
The overall energetics of the reaction is unfavorable. The delta-G is greater than null because joining two molecules creates more order.
How does the cell solve the second problem?
By coupling the unfavorable dehydration (or condensation) reaction to a very favorable
hydrolysis reaction, which is ATP + H2O -> ADP + Pc [Pc
PO43
The coupling goes like this: picture
These reactions are energetically favorable, but in the aqueous environment of the cell
not very likely for three reasons:
1. Glutamyl phosphate is very reactive and is very rapidly hydrolyzed
2. Ammonia (NH3) is in equilibrium with ammonium (NH4+). The ration of NH3:NH4+ at the pH of the cell ~7.2 is 1:107 and NH4+ will not work in this reaction.
3. There are many cometing side reactions, like the amino group on glutamic acid attacks the glutamyl phosphate. The product of this side reaction is useless to the cell and is not easily reversed.
How does the cell solve these problems?
By creating a molecular workbench (or cutting board) to hold the reactants still and in
the correct orientation in order for the reaction to proceed.
How can a molecular workbench be created?
How can one molecule hold on to another?
The molecular workbenches are specialized proteins called enzymes. They hold onto the
reactants or substrates weak, noncovalent bonds.
Van der Waals
ionic bonds
hydrogen bonds
hydrophobic effect
see panel 3-1 pp92,93 in alberts
Hydrogen bonds (H-bonds) play a key role in how molecules interact. H-bonds give water its
special solvent properties picture
H-bonds play an essential role in protein structure picture
How can protein become a molecular workbench?
Proteins are long polypeptide chains comprised of various arrangements of 20 naturally
occuring amino acids. The combined effects of the four weak noncovalent bonds help fold
the protein into a specific conformation. Theoretically there is a hugh number of possible
conformations. Only one is the active or native conformation. A variety of reagents (or
chemicals) or treatments can unfold or denature a protein. Removal of these conditions
allows the protein to refold to its native form.
1. H-bonds can form between two peptide bonds in the protein backbone, or between a peptide bond and a residue side chain, or between two side chains.
2. Non polar (or hydrophobic) residues move to the interior of the protein.
3. Ionic interactions of changed residues exist both on the surface and interior of the protein.
4. Disulfide bonds are a special class of covalent bonds that exist for secreted proteins, not cytoplasmic.
There are four levels of protein organization or structure:
1. Primary structure: the sequence of amino acids, which is read from the amino terminus
2. Secondary structure stable local regions of hbonded structures known as alpha helix and beta sheets, which
3. Tertiary structure the arrangement of 2 degree structures into specific arrangements or motifs or domains. The arrangement of domains within a single polypeptide is also considered tertiary structure
4. Quaternary structure is the combination of polypeptide chains to for multi-solvent structures.
Helices play special role in biology because they represent a very stable arrangement for polymeric structures.
How do the four levels of structure creat a molecular workbench?
The folding of the polypeptide creates a surface that can, through the forces of
evolution, conform to bind all the reactants or substrates of a reaction. For example to
hold onto glutamic acid the protein surface needs to look like this: picture
For the protein to hold onto ATP, the surface needs to look like this: picture
Evolution has conserved the ATP binding site. There are only a small number of amino acid
sequences that are known to bind atp.
By joining to glutamic acid binding domain, nature evolved glutamine synthase. Reading for
next class--Microscopy pp 139-156