 (II)
(II) Naturally occuring dioxygen carriers and storage proteins contain a transition metal
ion to which dioxygen can reversibly bind, eg. Iron (myoglobin,Mb, haemoglobin, Hb) or
Copper (haemocyanin). In this experiment a simple cobalt complex will be prepared which
also reversibly binds dioxygen. Many complexes of this type have been used as
"models" to aid in the understanding of how the proteins function.
When Co(salen) was first prepared in 1933, it was observed that the red-brown crystals
darkened on exposure to air. However, it was not until five years later that it was
established that the colour change was due to reversible uptake of dioxygen. H2salen
(I) is a Schiff-base ligand formed by the condensation of two molecules of salicylaldehyde
with 1,2-diaminoethane (ethylenediamine).
In 1944, it was found that different crystalline forms existed depending on the solvent
used in the preparation or for recystallisation and that these had varying capacity for
oxygenation in the solid state. This variation in oxygenation has been related to the
presence of voids in the crystal lattice, sufficient to allow the passage of molecular
oxygen. This suggestion is supported by the X-ray crystal structure determination of the
so-called "inactive" form which shows that the structure consists of di meric
units [CoSalen]2, (II).
 (II)
(II)
The active forms of Cosalen are presumed to contain dimeric units with open lattices packing relative to the inactive form. One form (III) has one Co atom directly above the other.
 (III)
(III)
The importance of solid-state packing effects in determining oxygenation ability is
further indicated by kinetic studies of dioxygen uptake at the crystal surfaces of the
different forms of Co(salen). Measurements involving different temperatures and pressure
conditions have shown that, following an induction period and after attainment of
equilibrium, the kinetics of dioxygen absorption at the surface were no longer important
and the uptake was controlled only by the rate of diffusion of dioxygen into the crystal.
In solution, it has been found that, depending on the solvent, in the absence of dioxygen,
the cobalt(II) may be four, five or six coordinate. For example, in a strongly
coordinating solvent such as pyridine, both [Co(salen).pyr] and [Co(salen).2pyr] exist,
whilst in chloroform, the major species appears to be Co(salen). Irrespective of the
solvent, the rate of dioxygen uptake appears to be similar, however the product obtained
may be a 1:1 (IV) or a 2:1 (V)(oxygen bridged) complex.
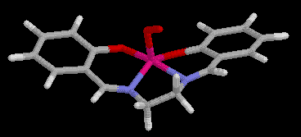 (IV)
(IV)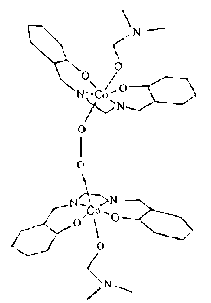 (V)
(V)
In this experiment, the inactive form of Co(salen) is prepared. The uptake of dioxygen is then investigated for the complex in DMSO solution to establish whether a 1:1 or a 2:1 complex is formed under these conditions.
To a solution of salicylaldehyde (2.1 cm3) in 25 cm3 boiling ethanol is added 1,2-diaminoethane (0.7 cm3). The reaction mixture is thoroughly stirred for 3-4 minutes and the solution then left to cool in an ice-bath. The bright yellow flaky crystals are filtered under suction and washed with a small volume of ice-cold ethanol, then air-dried. The yield and the melting-point should be recorded.
This preparation is sensitive to air, so should either be performed whilst flushing a
stream of nitrogen gas through the flask or under vacuum. The arrangement for working
under reduced pressure is given below.
Dissolve H2salen (1.6 g) in 60 cm3 of ethanol at 60░¸70░ŚC in a
side-arm flask fitted with a clamp. The side-arm is connected to an aspirator. Upon
dissolution of the ligand, quickly add Cobalt(II) acetate tetrahydrate (1.25 g) in 7 cm3
of ethanol:water mixture, while swirling the flask. Immediately stopper the flask and
evacuate through the side-arm for a short while. NOTE: The flask MUST be securely clamped
since the ethanol mixture has a tendency to bump vigorously. Be careful not to draw off
the bulk of the ethanol and clamp the side-arm once reduced pressure has been established.
Continue heating with periodic swirling for 1-2 hours.
During this period, the initially formed brown "active" complex slowly changes
to the brick-red "inactive" complex. Once this has occurred, cool the solution
to room temperature, collect the crystals on a sintered glass filter funnel (in air) and
wash three times with 7 cm3 of ice-cold ethanol. Dry in a dessicator, then
record the yield.
An IR spectrum from a typical student preparation is
available.
Accurately weigh out a sample of Co(Salen) which has been finely ground (between 0.05
and 0.1 g) and place it in a side-arm test tube. Transfer DMSO (approximately 5 cm3)
to a small beaker and bubble oxygen through it for a few seconds. (CAUTION: although DMSO
is not itself poisonous, it is readily absorbed by the skin and can easily carry other
compounds through the skin with it). Now transfer this DMSO to a small test tube that can
fit inside the side-arm test tube and lower the tube carefully inside without spillage.
Connect up the apparatus as shown in the diagram, so that the movable arm reservoir can be
adjusted to bring the water level of the graduated tube near the bottom.
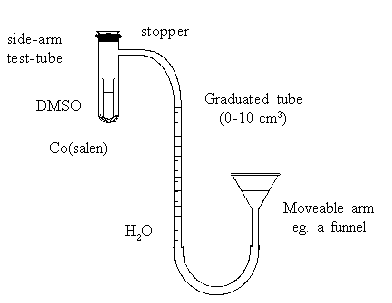
Flush the side-arm tube with a gentle stream of oxygen. Insert a tightly fitting rubber
stopper in the mouth of the tube. Adjust the movable arm to make the water levels equal in
both sides (ie ensuring that the pressure within the apparatus is atmospheric). Record the
water level in the graduated tube.
Finally, carefully invert the side-arm tube (holding near the stopper to minimise heating
by the hand) and record the time. The DMSO should be allowed to dissolve the Co(salen) but
not be spilled into the side-arm. As oxygen is absorbed, the water level in the graduated
tube begins to rise. Note the changes occurring in the tube. Continue shaking until no
further change in water level occurs (taking a reading every two minutes for 20 minutes is
usually sufficient). Adjust the moveable arm before each reading so that the water levels
in the tubes are again equal.
Draw a graph of volume changes versus time and extrapolate to estimate the overall total
oxygen uptake. From this volume change at room temperature and atmospheric pressure, the
number of mole of dioxygen absorbed per mole of Co(salen) can be calculated.
Suppose that an electronic spin system of S=0.5 is brought under the influence of a static magnetic field H. The energy state of the system would split into two, Ms=+0.5 and Ms=-0.5, owing to the interaction of the spin system with the magnetic field. The energy gap between the two states is DE = 2bH, where b is in Bohr Magnetons, the unit of electron spin moment. The majority of the spins of the system are in the lower energy state. When an electromagnetic wave of frequency v is applied to this system and a condition DE=hv is satisfied, an excitation from the lower to the upper level occurs. As a result, a fraction of the energy of the electromagnetic wave will be absorbed by this system. For a free unpaired spin then,
DH= hv=2bH
Usually a microwave of 3.2 cm wavelength (called the X-band) is used for EPR. Then v ~ 9200 MHz. By putting explicit values for b and h, we find that H =3200 gauss. The resonance condition is more generally expressed using the g value ie,
DH= hv=gbH
where g is called the spectroscopic splitting factor and is equal to 2.00229 for a free radical.Values of g for a formal orbitally singlet ground state can be expressed by;
g=2(1-nl/D)
where l is the effective spin-orbit coupling constant, D is the energy gap between the two states DE = 2bH