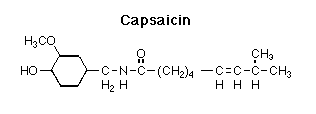
(For those with graphic-impaired viewers, an ascii version is also available)
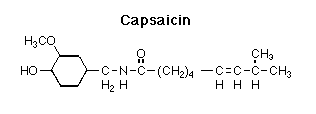
(For those with graphic-impaired viewers, an ascii version is
also available)
![]() Capsaicin, also known as
N-Vanillyl-8-methyl-6-(E)-noneamide, is the most pungent of the group of compounds called capsaicinoids
isolated from peppers. It is sparingly soluble in water, but very soluble in fats, oils
and alcohol. The second most common capsaicinoid is Dihydrocapsaicin:
Capsaicin, also known as
N-Vanillyl-8-methyl-6-(E)-noneamide, is the most pungent of the group of compounds called capsaicinoids
isolated from peppers. It is sparingly soluble in water, but very soluble in fats, oils
and alcohol. The second most common capsaicinoid is Dihydrocapsaicin:
![]() Capsaicin and Dihydrocapsaicin together
make up 80-90% of the capsaicinoids found in the fruit. In C. annuum the total
capsaicinoid content ranges from 0.1 to 1.0%, and the capsaicin:dihydrocapsaicin ratio is
about 1:1. In C. frutescens(Tabasco peppers) the total content ranges from 0.4-1.0%
with the ratio around 2:1.
Capsaicin and Dihydrocapsaicin together
make up 80-90% of the capsaicinoids found in the fruit. In C. annuum the total
capsaicinoid content ranges from 0.1 to 1.0%, and the capsaicin:dihydrocapsaicin ratio is
about 1:1. In C. frutescens(Tabasco peppers) the total content ranges from 0.4-1.0%
with the ratio around 2:1.
![]() The minor capsaicinoids include Nordihydrocapsaicin
[Dihydrocapsaicin with (CH2)5 instead of (CH2)6], Homocapsaicin [Capsaicin with
(CH2)5 instead of (CH2)4], and Homodihydrocapsaicin [Dihydrocapsaicin with (CH2)7
instead of (CH2)6].
The minor capsaicinoids include Nordihydrocapsaicin
[Dihydrocapsaicin with (CH2)5 instead of (CH2)6], Homocapsaicin [Capsaicin with
(CH2)5 instead of (CH2)4], and Homodihydrocapsaicin [Dihydrocapsaicin with (CH2)7
instead of (CH2)6].
![]() The pungencies of these five pure
compounds in Scoville Units (SU) are as follows:
The pungencies of these five pure
compounds in Scoville Units (SU) are as follows:
Compound Pungency x 100,000 SU
Capsaicin 160
Dihydrocapsaicin 160
Nordihydrocapsaicin 91
Homocapsaicin 86
Homodihydrocapsaicin. 86
From: Govindarajan, VS and Sathyanarayana, MN; Capsicum - Production, Technology, Chemistry and Quality. Part V. Impact on Physiology, Nutrition and Metabolism; Structure, Pungency, Pain and Desensitization Sequences, Crit. Rev. Food Sci. Nutr. 29, 435. 1991
![]() There are also 2 minor homologs and
three analogs with straight alkyl chains that exist in nature.
There are also 2 minor homologs and
three analogs with straight alkyl chains that exist in nature.
Chile-Heads created and copyright ©1995-1996 by Mike Bowers