The visible spectra in acid and base have been recorded and saved in JCAMP-DX format.
Work done in the Department on logwood dyes (1950's) to go here.
Structures of hematein and hematoxylin.
These are the water soluble compounds responsible for the red to blue colours in fruits and vegetables such as grapes, strawberries, apples, red cabbage, etc.
In general they are glycosides of anthocyanidins, where the sugars are usually glucose, galactose, rhamnose or arabinose. A Table showing the structures of 18 anthocyanidins is available.
The extinction coefficient for the anthocyanine dye E163 is reported for a 1% solution in 1 cm cell to be 1120 at 537 nm. A dilute solution gives the following spectrum, E163.
As much as 200 mg of betanin is found in beetroot. The red colour in Amanita muscaria is due to betanin as well.
For some background information on the cochineal insect see the report by Teresa Stoker.
The structure of carminic acid is available. Note
that both haematein and cochineal were mentioned by Robert Hooke in 1650 although they
both must have been known to the indigenous people of the Caribbean and Mexico for much
longer.
CurcuminCurcumin from turmeric is a yellow dye |
The RAMAN spectra of a number of prehistoric dyes and natural pigments have been
recorded and are available as GRAMS .spc files for download from University
College, London. Permission has been granted to convert two of these to JCAMP-DX
format, and they are available below, namely:
saffron and Prussian
Blue.
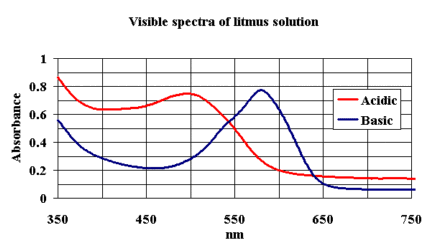
A starting point is "well known litmus", except that the structure is still
not well established since the solution used in laboratories is a mixture of perhaps more
than 6 species.

The visible spectra in acid and base have been recorded and saved in JCAMP-DX format.
The structures of some common acid base indicators along with the theory behind their use is presented at Imperial College.
Colouring matter as food additives
Created April 1997. Last modified 30th June-98.
URL http://wwwchem.uwimona.edu.jm:1104/lectures/dyes.html