>
> THE
INERTNESS OF OXYGEN
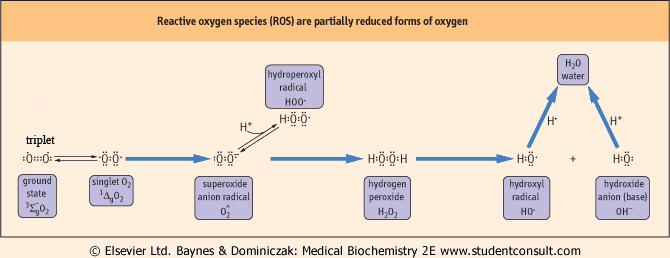
In most textbooks, oxygen is shown as a diatomic molecule with two bonds between the oxygen atoms. This is an attractive presentation, from the viewpoint of the electron dot structures and electron pairing to form chemical bonds, but it is incorrect. In fact, at body temperature, O2 is a biradical, a molecule with two unpaired electrons (Fig. 35.1). These electrons have parallel spins and are unpaired. Since most organic oxidation reactions, e.g. the oxidation of an alkane to an alcohol or an aldehyde to an acid, are two-electron oxidation reactions, O2 is generally not very reactive. In fact, O2 is completely stable in the presence of H2, a strong reducing agent, until enough heat is provided to flip an O2 electron and initiate the combustion reaction. Once it is started, the combustion provides the heat needed to propagate the reaction, sometimes explosively.
In most textbooks, oxygen is shown as a diatomic molecule with two bonds between the oxygen atoms. This is an attractive presentation, from the viewpoint of the electron dot structures and electron pairing to form chemical bonds, but it is incorrect. In fact, at body temperature, O2 is a biradical, a molecule with two unpaired electrons (Fig. 35.1). These electrons have parallel spins and are unpaired. Since most organic oxidation reactions, e.g. the oxidation of an alkane to an alcohol or an aldehyde to an acid, are two-electron oxidation reactions, O2 is generally not very reactive. In fact, O2 is completely stable in the presence of H2, a strong reducing agent, until enough heat is provided to flip an O2 electron and initiate the combustion reaction. Once it is started, the combustion provides the heat needed to propagate the reaction, sometimes explosively.
Copyright © 2007 Elsevier Inc. All rights reserved. Read our Terms and Conditions of Use and our Privacy Policy.
For problems or suggestions concerning this service, please contact: studentconsult.help@elsevier.com