Exposure to radiation from nuclear explosions or accidents, or breathing or ingestion of radioactive elements, such as Strontium-90 or radon gas, produces a flux of ROS in the body, causing mutations in DNA. Radiation therapy uses a focused beam of high-energy electrons or γ-rays from an X-ray or Cobalt-60 source to destroy tumor tissue. The radiation produces a flux of hydroxyl radicals (from water) and organic radicals at the site of the tumor, oxidizing and destroying the DNA of the tumor cell. Irradiation of food is also used as a method of sterilization to destroy bacterial and viral contaminants or destroy insect infestations in order preserve food products during long-term storage.
Metabolic reactions are conducted at body temperature, far below the temperature required to activate free oxygen. In biological redox reactions involving O2, the oxygen is activated by redox active metal ions, such as iron and copper. All enzymes that use O2 in vivo are metalloenzymes and, in fact, even the oxygen transport proteins, hemoglobin and myoglobin, contain iron in the form of heme. These metal ions provide one electron at a time to oxygen, activating O2 for metabolism. Because iron and copper, and sometimes manganese
ISCHEMIA/REPERFUSION INJURY A patient suffered a severe myocardial infarction, which was treated with tissue plasminogen activator, a clot-dissolving (thrombolytic) enzyme. During the days following hospitalization, the patient experienced palpitations, irregular rapid heartbeat, associated with weakness and faintness. The patient was treated with anti-arrhythmic agents.
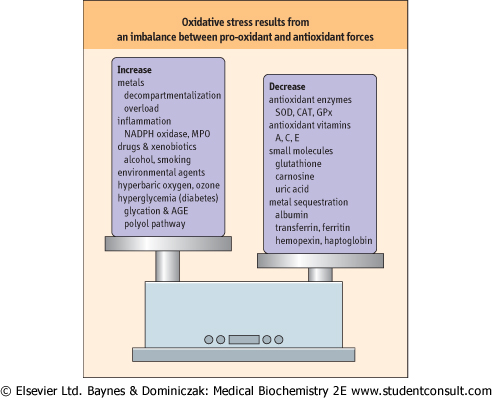 Figure 35.2 Oxidative stress: an imbalance between pro-oxidant and
antioxidant systems.
As described in this chapter, numerous factors contribute to the
enhancement and inhibition of oxidative stress. AGE, advanced glycation
end-product; CAT, catalase; GPx, glutathione peroxidase; MPO,
myeloperoxidase.
Figure 35.2 Oxidative stress: an imbalance between pro-oxidant and
antioxidant systems.
As described in this chapter, numerous factors contribute to the
enhancement and inhibition of oxidative stress. AGE, advanced glycation
end-product; CAT, catalase; GPx, glutathione peroxidase; MPO,
myeloperoxidase.Copyright © 2007 Elsevier Inc. All rights reserved. Read our Terms and Conditions of Use and our Privacy Policy.
For problems or suggestions concerning this service, please contact: studentconsult.help@elsevier.com