The Gibbs' free energy (ΔG) of a reaction is the maximum amount of energy that can be obtained from a reaction at constant temperature and pressure. The units of free energy are kcal/mol (kJ/mol). It is not possible to measure the absolute free energy content of a substance directly, but when reactant A reacts to form product B, the free energy change in this reaction ΔG, can be determined. For the reaction A → B:
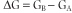 where GA and GB are the free energy of A and B, respectively. All reactions in biologic systems are considered to be reversible reactions, so that the free energy of the reverse reaction, B → A, is numerically equivalent, but opposite in sign to that of the forward reaction.
where GA and GB are the free energy of A and B, respectively. All reactions in biologic systems are considered to be reversible reactions, so that the free energy of the reverse reaction, B → A, is numerically equivalent, but opposite in sign to that of the forward reaction.
|
| It is clear that, if there is a greater concentration of B than of A at equilibrium, the reaction A → B is favorable - that is, it tends to move forward from a standard state in which A and B are present at equal concentrations. In this case, the reaction is said to be a spontaneous or exergonic reaction, and the free energy of this reaction is defined as negative: that is, ΔG < 0, indicating that energy is liberated by the reaction. Conversely, if the concentration of A is greater than that of B at equilibrium, the forward reaction is termed unfavorable, nonspontaneous or endergonic, and the reaction has a positive free energy: that is, B tends to form A, rather than A to form B. In this case, energy input would be required to push the reaction A → B forward from its equilibrium position to the standard state in which A and B are present at equal concentrations. The total free energy available from a reaction depends on both its tendency to proceed forward from the standard state (ΔG) and the amount (moles) of reactant converted to product.
|
| The free energy of metabolic reactions is related to their equilibrium constants
|
Thermodynamic measurements are based on standard-state conditions where reactant and product are present at 1 molar concentrations, the pressure of all gases is 1 atmosphere and the temperature is 25°C (298°K). Most commonly, the concentrations of reactants and products are then measured after equilibrium is attained. Standard free energies are represented by the symbol ΔG° and biological standard free energy change by ΔG°', with the accent symbol designating pH 7.0. The free energy available from a reaction, may be calculated from its equilibrium constant by the Gibbs equation:
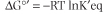 where T is absolute temperature (°Kelvin), lnKeq is the natural logarithm of the equilibrium constant for the reaction, and R is the gas constant:
where T is absolute temperature (°Kelvin), lnKeq is the natural logarithm of the equilibrium constant for the reaction, and R is the gas constant:
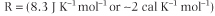
|
Several common metabolic intermediates that you will encounter in your studies are listed in Table 8.2, along with the equilibrium constants and free energies for their hydrolysis reactions. Those intermediates with free energy changes equal to or greater than that of ATP, the central energy transducer of the cell, are considered to be high-energy compounds, and generally have either anhydride or thioester bonds. The lower-energy compounds listed are all phosphate esters and, in comparison, do not yield as much free energy on hydrolysis. The hydrolysis reaction of glucose-6-phosphate (Glc-6-P) is written as:
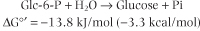 This reaction has a negative free energy and occurs spontaneously. The reverse reaction, synthesis of Glc-6-P from glucose
This reaction has a negative free energy and occurs spontaneously. The reverse reaction, synthesis of Glc-6-P from glucose and phosphate, would require input of energy. and phosphate, would require input of energy.
|
| page 94 |  | | page 95 |
|
Table 8-2.
Thermodynamics of hydrolysis reactions. |
| Body_ID: None |
| Thermodynamics of hydrolysis reactions |
| Body_ID: T008002.50 |
| Metabolite | K'eq | ΔG°' (kJmol-1) | (kcalmol-1) |
| Body_ID: T008002.100 |
| phosphoenolpyruvate | 1.2 × 1011 | -61.8 | -14.8 |
| Body_ID: T008002.150 |
| Phosphocreatine | 9.6 × 108 | -50.2 | -12.0 |
| Body_ID: T008002.200 |
| 1,3-bisphosphoglycerate | 6.8 × 108 | -49.3 | -11.8 |
| Body_ID: T008002.250 |
| Pyrophosphate | 9.7 × 105 | -33.4 | -8.0 |
| Body_ID: T008002.300 |
| acetyl coenzyme A | 4.1 × 105 | -31.3 | -7.5 |
| Body_ID: T008002.350 |
| ATP | 2.9 × 105 | -30.5 | -7.3 |
| Body_ID: T008002.400 |
| glucose-1-phosphate | 5.5 × 103 | -20.9 | -5.0 |
| Body_ID: T008002.450 |
| fructose-6-phosphate | 7.0 × 102 | -15.9 | -3.8 |
| Body_ID: T008002.500 |
| glucose-6-phosphate | 3.0 × 102 | -13.8 | -3.3 |
| Body_ID: T008002.550 |
|
| Body_ID: T008002.600 |
Equilibrium constants and free energy of hydrolysis of various metabolic intermediates at pH 7 (ΔG0')
|
|