| page 333 |  | | page 334 |
| Figure 23.1 Acid-base balance. Lungs, kidneys, and erythrocytes contribute to the maintenance of acid-base balance. The lungs control gas exchange with the atmospheric air. Carbon dioxide generated in tissues is transported in plasma as bicarbonate; the erythrocyte hemoglobin (Hb) also contributes to CO2 transport. Hemoglobin buffers hydrogen ion derived from carbonic acid. The kidneys reabsorb filtered bicarbonate in the proximal tubules and generate new bicarbonate in the distal tubules, where there is a net secretion of hydrogen ion. |
| The bicarbonate buffer is unique, because it remains in equilibrium with atmospheric air, creating an open system with
many times greater capacity than that of any of the 'closed' buffer systems. This is how it works:
|
Carbon dioxide produced in tissues diffuses through cell membranes and dissolves in plasma. The solubility coefficient of CO2 in plasma is 0.23 if pCO2 is measured in kPa (or 0.03 if pCO2 is measured in mmHg; 1 kPa = 7.5 mmHg or 1 mmHg = 0.133 kPa). Thus, at normal pCO2 of 5.3 kPa (40 mmHg), the concentration of dissolved CO2 (dCO2) is:
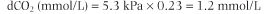
|
| RESPIRATORY AND METABOLIC COMPENSATION IN ACID-BASE DISORDERS |
| Respiratory and metabolic components of the acid-base balance adjust to maintain optimal blood pH. When the primary disorder is respiratory, the accumulation of CO2 leads to a compensatory increase in bicarbonate reabsorption by the kidney. Conversely, a decrease in pCO2 decreases bicarbonate reabsorption. In the event that the primary disorder is metabolic, a decrease in bicarbonate concentration and resulting decrease in pH stimulate the respiratory center to increase the ventilation rate: CO2 is blown off and pCO2 in plasma decreases. This is why patients with metabolic acidosis hyperventilate. Conversely, an increase in plasma bicarbonate concentration, leads to an increase in pH and to a decrease in the ventilation rate: CO2 is retained, and the pH returns towards normal. |
|
Table 23-1.
Main buffers in the human body. |
| Body_ID: None |
| Buffers in the human body |
| Body_ID: T023001.50 |
| Buffer | Acid | Conjugate base | Main buffering action |
| Body_ID: T023001.100 |
| Hemoglobin | HHb | Hb- | erythrocyles |
| Body_ID: T023001.150 |
| Proteins | HProt | Prot- | intracellular |
| Body_ID: T023001.200 |
| Phosphate buffer |
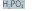 |
 | intracellular |
| Body_ID: T023001.250 |
| Bicarbonate | CO2 → H2CO3 |
 | extracellular |
| Body_ID: T023001.300 |
|
| Body_ID: T023001.350 |
(For the principles of buffering action see Chapter 2)
|
| This CO2 equilibrates with H2CO3 in plasma in the course of a very slow, nonenzymatic reaction. As a result there is only very little, about 0.0017 mmol/L, of H2CO3 normally present in plasma. However, because of equilibrium between the formed H2CO3 and dissolved CO2 (theoretically all dissolved CO2 could eventually convert into H2CO3), we take the 'acid' component of the bicarbonate buffer as being equal to the plasma concentration of dissolved CO2.
|
The Henderson-Hasselbalch equation (see Chapter 2) expresses the relationship between pH and the components of the bicarbonate buffer. It reads:
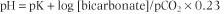
|
| Thus the plasma pH is determined by the ratio between the concentrations of plasma bicarbonate (the 'base' component of the buffer) and the dissolved CO2 (the 'acid' component).
|
| page 334 |  | | page 335 |
Normally, at a plasma pCO2 of 5.3 kPa (dCO2 concentration 1.2 mmol/L), the erythrocytes and renal tubular cells
maintain the plasma bicarbonate concentration at about 24 mmol/L. The pK (see Chapter 2) of the bicarbonate buffer is 6.1, and if we insert the concentrations of buffer components into the Henderson-Hasselbalch equation above the pH is very near 7.40 (hydrogen ion concentration 40 nmol/L)
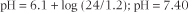
|
| Thus, pH 7.40 is the average pH of the extracellular fluid at a normal concentration of bicarbonate and normal partial pressure of CO2.
|
The bicarbonate buffer minimizes changes in hydrogen ion concentration when either acid or alkali are added to blood. This is how it works: When an acid (H+) is added, it reacts with bicarbonate; this leads to a release of CO2. Thus pCO2 in blood increases slightly and excess of CO2 is subsequently eliminated through the lungs:
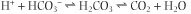
|
When alkali (OH-) is added, carbonic acid/CO2 neutralizes it to water, and the bicarbonate concentration in plasma increases slightly.
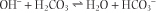 as a consequence of a decrease in the H2CO3 concentration; the reaction
as a consequence of a decrease in the H2CO3 concentration; the reaction
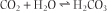 proceeds to the right, decreasing pCO2
proceeds to the right, decreasing pCO2
|
| The depletion of CO2 is subsequently compensated by a decreased ventilation rate and its resulting retention in blood. The above shows that the denominator in the Henderson-Hasselbalch equation is controlled by the lungs. This is why we call pCO2 'the respiratory component of the acid-base balance'.
|
On the other hand, plasma bicarbonate concentration is controlled by the kidneys and erythrocytes (therefore we call the bicarbonate concentration 'the metabolic component of the acid-base balance'). Erythrocytes and renal tubular cells contain a high activity of a zinc-containing enzyme, carbonic anhydrase (CA; also known as carbonic dehydratase), which catalyses the conversion of dissolved CO2 into carbonic acid:
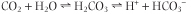
|
| However, the carbonic acid dissociates, forming the hydrogen and bicarbonate ions. Renal tubular cells and erythrocytes are therefore sources of bicarbonate. Through this reaction the kidneys control plasma bicarbonate concentration by regulating its reabsorption and synthesis, and the erythrocytes make fine adjustments to the plasma bicarbonate concentration in response to changes in pCO2.
|
|