Free Radicals:
An atom or a group of atoms with an unpaired electrons both side of peroxide ![]() H2O2
H2O2![]() oxygens.
oxygens.
Radicals are unusually reactive. Peroxide molecule as radical 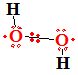 two free electrons
two free electrons
on repulsing orbitals compensate two binding orbitals between oxygen atoms. Peroxide
molecules are strongly per-oxidizing species capable causing a wide range of damage in life bodies
for protein polymer molecules, nucleic acid polymer molecules, carbohydrates polymer molecules,
lipids like as fatty acids, steroid hormons and holestrol molecules
in biological systems as they are life bodies.
Back to the catalase tutorial.