Chemical equilibrium disruption processes in Nature,
environment transformation and
importance of Le Chatelier theorem discovery for Life HOMEOSTASIS and
technologies
Āris
Kaksis, 2015. year, Riga Stradin`s University
Global
observations last hundred years confirm warming of planet or increase
surface temperature per
0.5° degrees of planet Earth, what accompanies
environmental changes. For example, storms or storming
cyclones
intensity and frequency, increases carbonic dioxide or carbonic(IV)
oxide concentration in air from
0,02 % volume fractions in 1900 year to
0,038% volume fractions in 2007 year.
Draining
from reaction medium heat (cooling, what usually describe with
decreasing of temperature)
has shifts the equilibrium to direction of
exothermic reaction or reverse way adding heat shifts the equilibrium
to direction of endothermic reaction, et cetera in air is evolving the CO2 .
In many
discussions about chemical equilibrium nature is known example, that
exist equilibrium
between carbon(IV) oxide and in water dissociated
bicarbonate anion with hydrogen ions. CO2
gas dissolution
reaction is exothermic therefore warming the
planet shifts equilibrium towards CO2
gas evolving direction,
because heat adding shifts equilibrium
to endothermic reaction. As well planet and oceans warming increase
CO2 air concentration.
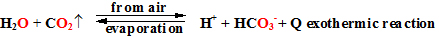
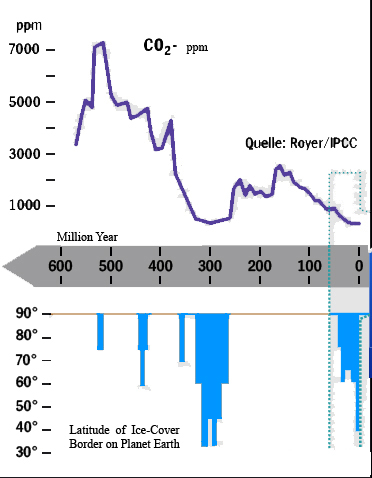
Reconstruction climate of Earth shows temperature and
CO2 oscillation, which 600
million years back in historical 10000
years period is observing earth
warming from -50° to 45° degrees and carbon(IV) oxide gas concentration
in air reaches 60%,
what corresponds 600000 ppm.
As is seen climate
changes
are occurring with corresponding chemical equilibrium
disarranging and new equilibrium establishing,
what we can observe as CO2 concentration changes in air.
|
|
Before
600 million years CO2 concentration
in atmosphere approximately like present-day 0.03% volume fractions,
what agree 300 ppm.
Atmospheric oxygen O2 concentration
approximately 1% from today’s 20.95% volume fraction was unconformable
for bulk of present-day animal species.
Approximately 600 million years back Earth was ice
covered reminding Snowball Earth. Glacier fast melting provokes CO2 concentration growth up to 60% -
600000 ppm.
Due to
greenhouse effect temperature increases from -50° up to 45° degrees,
what because of photosynthetic reaction brought oxygen O2 concentration fast increase above
present-day 20.95% up to 30% volume fractions, and established oxygen
O2 content level in
atmosphere 20.95% volume fraction for next 500 million years.
During the
last 2.6 million years or so in the
Quaternary
period, ice ages, also called glacial ages, were times of extreme
cooling of the Earth's climate where ice sheets and
other types of glacier
expanded to cover large areas of land. Between ice ages there were warmer
interglacial periods and we are now living during such a time.
There have been many ice ages during the last 2.6 million years but when people talk about the |
Ice Age, they are often referring to the most recent glacial period, which start 13557 years ago and ended about
11,500 years ago and preceding warmer interglacial period end at 13557 years because as written in Indians,
Egyptians, Assyrians and Mayan calendar, the preceding proto civilization was go waste on data 11542 year B.C.
and refers to days civilization before 13557 years. What causes ice ages is not completely understood.
The composition of the atmosphere, changes in the position of our planet around the Sun, changes in ocean
currents and the Sun radiation as spot activity irregular as well as regular (11 year periods) changes are some
of the important factors that control the climate. Exist else unknown factors influence on climate? Sun and Earth
increase! Earth radius in 3000 years increases per 60 km, Mayan astronomers and to days since measure.

Image:
A reconstruction of the Anglian ice sheet in Precambrian
North London (credit: The Natural
History Museum, London) CO2 60% and warming increased
temperature shifts photo-synthesis
towards → C6H12O6 + 6 O2. Oxygena O2 concentrationa in atmosphere
reaches 35% volume fraction
amount that relevente over 20,95%.
PreCembrian
Ediacaran period 635 – 545 million years ago
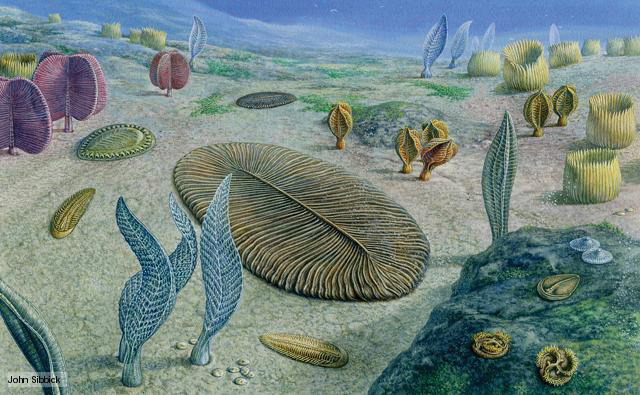
Known
also as the Vendian, the Ediacaran was the final stage of Pre-Cambrian
time. All life in the Ediacaran
was soft-bodied - there were no bones,
shells, teeth or other hard parts. As soft bodies don't fossilise very
well, remains from this period are rare. The world's first ever
burrowing animals evolved in the Ediacaran,
though we don't know what
they looked like. The only fossils that have been found are of the
burrows
themselves, not the creatures that made them. This period gets
its name from the Ediacara
Hills in Australia,
where famous fossils of
this age were found.
Cambrian
period 545 – 495 million years ago

The
Cambrian is famed for its explosion of abundant and diverse life forms.
Life had diversified into many
forms and many ways of living: animals
now swam, crawled, burrowed, hunted, defended themselves and
hid away.
Some creatures had evolved hard parts such as shells, which readily
fossilised and left a clear
record behind. However, sometimes
geologists get lucky and find beautiful fossils of soft and squishy
creatures - as at the Burgess Shale site. In Cambrian times there was
no life
on land and little or none in
freshwater - the sea was still very
much
the centre of living activity.
Carbon dioxide concentration
increase on air change global equilibrium for green plants
photosynthetic
reaction: (red and blue light photon energy
absorption)
glucose
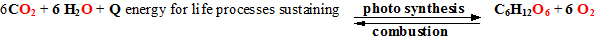
In
global equilibrium of life nature organisms are imagine expressed as
glucose (C6H12O6) and
oxygen (O2) „combusted” transforming
reverse way to water and carbon dioxide. So cells and our organisms
use
in photosynthetic reaction accumulated energy of red and blue light
photons. In life nature occurring is
reverse reaction of photosynthesis
„combustion”, of which evolved energy is used for our organism warming
and maintenance the life processes in body.
Investigations shows that 600 millions year ago in 10000 year period
was observing increased oxygen concentration
on air 30% volume
fractions comparing with present-day 20,95% volume fractions. Higher
temperature and
greater oxygen concentration accelerate evolution of
animals on Earth and scientists think, that it promote
multi cellular
organisms and humans birth on planet Earth.
Scientists
in investigations have clear up, that ordinary plant species existence
is possible if carbon
dioxide CO2
concentration in air do not drops below 0,005% volume fraction 50 ppm,
other way green plants on
Earth perish due to insufficient material CO2 resources, which dramatically would
lead to oxygen O2
extinction on planet
Earth atmosphere and for us not to be what to breath and after all also
what to eat,
because no more glucose C6H12O6
created, however on Earth such risk of evolution never have been exist.
4 oxygen O2 with 4H+ from water medium adsorb deoxy T hemoglobin HbT of inspired fresh air.
4O2+4H++(His63,58)4HbTbetaVal1(NH4+PO42-G-)2<=>(H+His63,58)4Arg+His+betaVal1(NH4+)2HbR(O2)4+BPG5-
H2COPO32--HCOPO32--COO->BPG5- is glycerate dihydroxy acid salt G- of two phosphate
2,3-esters with homeostasis concentration [BPG5-]= 5 mM and is glycolysis metabolite in erythrocytes
which regulate [O2] concentration sensitive equilibrium shift to turn transition oxy R=>deoxy T
at lowered concentration below [O2]=6•10-5M in blood plasma because of BPG5- squeeze in to cavity
due to oxygen pressure decrease as desorbs four oxygens 4 O2, and four 4 H+ following equilibrium shifts
to right oxy R=>deoxy T .
(H+His63,58)4Arg+His+betaVal1(NH4+)2HbR(O2)4+BPG5- <=>4O2+4H++(His63,58)4HbTbetaVal1(NH4+PO42-)2G-
Arterial blood homeostasis oxygen concentration is [O2]=6•10-5M which [O2] reaching tissue cells decreases below
[O2]<6•10-5 M , therefore BPG5- squeeze in to cavity Oxygen 4 O2 desorbing from oxy HbR(O2)4 supply into
solution 4H+. Lung↔tissue Homeostasis venous deoxy HbT desorbed Oxygen O2 from hemoglobin acidify water
medium with 4H+, promoting breathe out CO2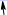 . Respiration one CO2 molecule evolving supports in water medium
. Respiration one CO2 molecule evolving supports in water medium
one proton H+, which desorbed form oxy HbR(O2)4 distal His63,58 histidines. Each H+ ion in respiration out CO2
shifts equilibrium to right side: 4H+ +4HCO3-+ 4Q<=>4H2O +4CO2 and pH remains constant in blood pH = 7,36 ,
and pH remains constant in blood pH = 7,36 ,
as one bicarbonate ion and one hydrogen ion produce one CO2 right side.
cytosol => => epithelial cell surface
H2CO3+ Q=H2O +CO2 |
4H+ concentration increase in lungs? That shifts bicarbonate HCO3- transport through membrane with CO2breezing out. As it shows blood buffer system physiological mechanism study for transport of protons H+ and bicarbonate HCO3-ions crossing membrane channels on alveolar epithelia cell surface.
How does work Le Chatelier’s principles in equilibrium of oxygen O2 inspiration and breathe out CO2in Homeostasis |
H++HCO3-+ Q endothermic<=>H2O +CO2 initial amount, concentration increase elevate CO2
initial amount, concentration increase elevate CO2 output.
output.
1) heating + Q shifts equilibrium right side => ;
2) hydrogenH+ion concentration (acidity) increase shifts equilibrium right side => Hb adsorbed O2 yield H+;
3) bicarbonate HCO3-concentration increase shifts equilibrium right side =>.
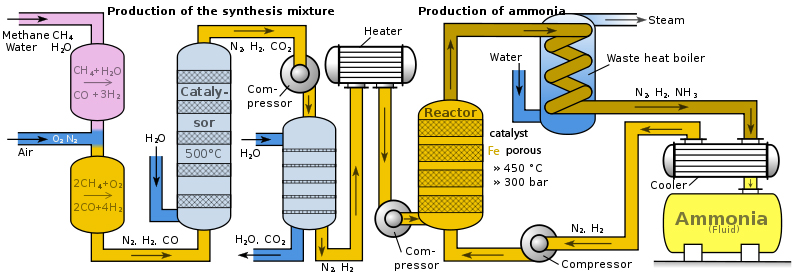
Ammoniac
can obtain in hydrogen reaction with nitrogen using catalyst
porous iron Fe. At absence of catalyst reaction
practically does not happen and has not established equilibrium.
Therefore if hydrogen run out in air ammoniac not forms and only stand
for dangerous explosion, because hydrogen reacts with oxygen if it
ignite. In oil, gas and coal refining industry arises huge amount of
hydrogen H2, as well hydrogen atoms containing organic compounds was
heated. Up to1920 year in refinement factories hydrogen was combusted
and hydrogen flame torches was factory landscape integral part, because
accumulation of hydrogen mixture with oxygen is dangerous explosive.
Haber invents device for ammoniac obtaining and in 1920 year on USA and
UK oil refinement factories was mounted first equipments. Le Chatlier
theorem has allowed up to optimal circumstances to
develop ammoniac obtaining technology. Equilibrium influences
temperature, pressure. Product pressure of ammoniac diminishes,
condensation into liquid due to cooling or dissolution into water. In
Haber process circulate two gases nitrogen N2
and hydrogen 3H
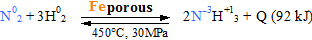
Initially equipment durability allowed 100 MPa pressure and 200° C
temperature, but at modern equipment optimal established 450° C
temperature and 30 MPa pressure. As catalyst uses porous iron Fe. Obtained equilibrium mixture contains 98%
ammoniac. Condensed NH3
in next box is made in water, in which dissolves NH3,
or condensed and liquid product feels in transport tank. Ammoniac gas
pressure decreasing pNH3↓
shifts equilibrium to right. Unused gases N2
+ 3H2 returns in porous iron Fe
reaction box and Haber cycle equilibrium established again during one
second with 98% ammoniac volume fraction. To remove oxygen from air (N2,O2), mixture introduces in Bosh process
together with methane and water (CH4, H2O) and obtains pure nit`rogen and
hydrogen mixture. Heated 450° C nitrogen and hydrogen mixture (N2 + 3H2) compressed
introduces in Haber cycle reactor , but Haber process rest of mixture (N2 + 3H2)
returns in reactor repeating reaction in technological cycle:
 ;
; 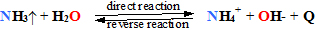
1. Gas product NH3↑
concentration is diminished dissolving in water or condensing liquid,
equilibrium shifts to product NH3
right and NH3 outcome
increases;
2. Increasing pressure above 30 MPa equilibrium shifts to left and
product NH3 outcome
decreases;
3. Decreasing pressure below 30 MPa decreases velocity of reaction on
catalyst porous iron Fe surface and
product
NH3 gain decreases;
4. Increasing temperature above 450° C degrees, equilibrium shifts to
direction of endothermic reaction to left, towards initial compounds N2 + 3H2 and product NH3 yield decreases;
5. Decreasing temperature below 450° C degrees, equilibrium shifts to
direction of exothermic reaction to right, towards product NH3 and yield increases, but
decreases reaction velocity on catalyst porous iron Fe surface and product NH3 yield decreases
On year 1990 in USA have produced 50 million tons ammoniac. Ammoniac is
nitrogen source for fertilizers in agricultural industry, because
ammoniac is resource for nitric acid HNO3 manufacturing, but from nitric acid
obtains nitric salts, which in agriculture industry designate with name
salpeter. On first half 20th century Chile exports salpeter of Chile
NaNO3
and from India purchased salpeter of India KNO3.
Those resources exhaust in former century, which replaces Haber cyclic
process technology introducing on oil and gas refinement factories.
Ammoniac solution in water call about ammoniac water.
Ammoniac very good dissolves in water. In medicine shops can to
purchase liquid ammonia (smelling salts), what is ammoniac solution in
water :
Life species on surface of Earth ozone layer cover from ultraviolet
radiation of Sun. Ozone molecules O3 forms
on high layers of atmosphere 10 to 35 kilometers high. Ultraviolet
radiation brakes double bond of oxygen molecule O=O, because collision energy is
sufficient for overcome energy barrier in reaction, that crack covalent
bonds:  + Q energy (ultraviolet radiation)
+ Q energy (ultraviolet radiation)  O + O
O + O
Possibility, that split oxygen atoms met each other is negligible
small, therefore reverse reaction velocity is very slow due to low
oxygen atoms O concentration and possible is
collision with other molecule of oxygen So forms ozone: O2 + O O3.
Overall reaction of equilibrium is performing as formation of two ozone
molecules
O3.
Overall reaction of equilibrium is performing as formation of two ozone
molecules
 + Q
energy (ultraviolet radiation) +2 O2
+ Q
energy (ultraviolet radiation) +2 O2  O3 + O3 .
O3 + O3 .
Equilibrium shifts towards ozone formation, if increases oxygen
concentration and ultraviolet radiation supplied amount of energy Q.
Any
compound, which react with ozone, dismantles ozone natural formation
equilibrium in higher atmosphere layers and ozone concentration
decreases, because ozone is depleted.
Ozone
forms in devices, which are mounted with ultraviolet lamps (copyist,
sanitary junctions of clinics, biological laboratories, agro cultural
technologies and sterilization rooms). Ozone forms in electric
discharges of sparkles. For example, oxygen ozonator of Riga water
refinement and in time of thunder storm. If on air in electric
discharge from nitrogen and oxygen forms nitric(II) oxide:
O2 + N2
 2 NO
, which react with oxygen:
2 NO
, which react with oxygen:
O2 + NO  2 NO2 + O and
atomic oxygen forms ozone:O2
+ O
2 NO2 + O and
atomic oxygen forms ozone:O2
+ O  O3
.
O3
.
Nitric(II) oxide also react with ozone : O3
+ NO  NO2
+ O2 and ozone
reacting out to converts about oxygen and nitric(IV) oxide.
NO2
+ O2 and ozone
reacting out to converts about oxygen and nitric(IV) oxide.
Thunder storm rain is fertile, because it makes richer soil with nitric
oxides (NO2, NO) performing nitrates, which are
valuable resource in plants life. If soil is richer with nitrates, then
healthy and darker green looks plants.
Chemical equilibrium disruption processes in Nature,
environment transformation and
importance of Le Chatlier theorem discovery for sciences and
technologies
1. Warming of planet Earth increases carbonic dioxide gas CO2 concentration in air.
2. Carbonic dioxide gas concentration growth shifts equilibrium for
green plant photosynthetic reaction to product formation and increases
in reaction produced glucose C6H12O6
and oxygenO2 amount.
3. Catalysts do not change reaction equilibrium state:
concentration, pressure and temperature influence on equilibrium, but
increases velocity for establishing of equilibrium.
4. Haber for ammoniac synthesis cycle found optimal circumstances,
applying Le Chatlier theorem and determined catalyst
, which increases velocity for establishing of equilibrium.
5. Ozone equilibrium on upper atmospheric layers depends on
compounds, which react out with ozone and so decreasing ozone
concentration 10 to 15 kilometer high.
![]() ;
; ![]()
![]() + Q energy (ultraviolet radiation)
+ Q energy (ultraviolet radiation) ![]() O + O
O + O![]() O3.
Overall reaction of equilibrium is performing as formation of two ozone
molecules
O3.
Overall reaction of equilibrium is performing as formation of two ozone
molecules ![]() + Q
energy (ultraviolet radiation) +2 O2
+ Q
energy (ultraviolet radiation) +2 O2 ![]() O3 + O3 .
O3 + O3 . ![]() 2 NO
, which react with oxygen:
2 NO
, which react with oxygen:![]() 2 NO2 + O and
atomic oxygen forms ozone:O2
+ O
2 NO2 + O and
atomic oxygen forms ozone:O2
+ O ![]() O3
.
O3
.![]() NO2
+ O2 and ozone
reacting out to converts about oxygen and nitric(IV) oxide.
NO2
+ O2 and ozone
reacting out to converts about oxygen and nitric(IV) oxide. 




