| Complex III - Cytochrome c reductase
|
| page 101 |  | | page 102 |
| Figure 8.8 Coenzyme Q10, or ubiquinone, accepts one or two electrons, transferring them from flavoproteins to Complex III. The semiquinone form is a free radical. |
| Cytochromes, found in the mitochondrion and endoplasmic reticulum, are proteins that contain heme groups, but which are not involved in oxygen transport (Fig. 8.9). The core structure of heme groups is a tetrapyrrole ring similar to that of hemoglobin, differing only in the composition of the side chains. The heme group of cytochromes b and c1 is known as iron protoporphyrin IX and is the same heme that is found in hemoglobin, myoglobin, and catalase. Cytochrome c contains heme C, covalently bound to the protein through cysteine residues. Cytochromes a and a3 contain heme A, which, in common with ubiquinone, contains an isoprene side chain. In hemoglobin and myoglobin, heme must remain in the ferrous (Fe2+) state; in cytochromes, the heme iron is reversibly reduced and oxidized between the Fe2+ and Fe3+ states as electrons are shuttled from one protein to another. |
This enzyme complex, also known as ubiquinone-cytochrome c reductase or QH2-cytochrome c reductase, oxidizes ubiquinone and reduces cytochrome c (Fig. 8.8). Reduced ubiquinone funnels electrons that it gathers from mitochondrial flavoproteins and transfers them to complex III. Electrons from ubiquinone are transferred through two species of cytochrome b, to an FeS center, to cytochrome c1, and finally to cytochrome c. Transport of two electrons to cytochrome c yields sufficient free energy change and protons pumped to synthesize about one mole of ATP. The overall reaction is:
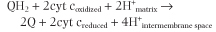
|
| Four protons are pumped, two from fully reduced ubiquinone and two from the matrix.
|
|