| The second major step in fatty acid biosynthesis also involves a multienzyme complex, fatty acid synthase.
|
This enzyme system is more complex than acetyl-CoA carboxylase. The protein contains seven distinct enzyme activities and an acyl-carrier protein (ACP). ACP, a peptide containing 77 amino acids , replaces the CoA as an entity which binds the growing fatty acid chain. It contains a pantetheine residue, the same as CoA. The relatively long pantetheine group acts as a flexible 'arm' which makes the molecule being synthesized available to different enzymes in the fatty acid synthase complex. The structure of the fatty acid synthase is shown in Figure 15.2: it consists of a dimer of large identical polypeptides (260 kDa each) arranged head to tail. Each chain contains all seven enzyme activities grouped into three domains; however, the function in fatty acid synthesis is shared between the two polypeptide chains. , replaces the CoA as an entity which binds the growing fatty acid chain. It contains a pantetheine residue, the same as CoA. The relatively long pantetheine group acts as a flexible 'arm' which makes the molecule being synthesized available to different enzymes in the fatty acid synthase complex. The structure of the fatty acid synthase is shown in Figure 15.2: it consists of a dimer of large identical polypeptides (260 kDa each) arranged head to tail. Each chain contains all seven enzyme activities grouped into three domains; however, the function in fatty acid synthesis is shared between the two polypeptide chains.
|
| The fatty acid synthase builds up the fatty acid molecule up to the 16-carbon length
|
| page 200 |  | | page 201 |
| Figure 15.2 Structure of fatty acyl synthase. Fatty acid synthase contains seven distinct enzyme activities and an acyl-carrier protein (ACP). Cys, cysteine. |
| The reaction proceeds after an initial priming of the cysteine (-Cys-SH) group with acetyl-CoA under the action of acetyl transacylase (Fig. 15.3). Malonyl-CoA is then transfered to the -SH residue of the pantetheine group attached to the ACP of the other subunit, under the action of malonyl transacylase. Next, 3-ketoacyl synthase (the condensing enzyme) catalyzes the reaction between the previously attached acetyl group and the malonyl residue, liberating carbon dioxide and forming the 3-ketoacyl-enzyme complex. This frees the
cysteine residue that had been occupied by acetyl-CoA. The 3-ketoacyl group subsequently undergoes sequential reduction, dehydration, and again reduction to form a saturated acyl-enzyme complex. The next molecule of malonyl-CoA then displaces the acyl group from the pantetheine-SH group to the now free cysteine group, and the reaction sequence is repeated through six cycles. Once the 16-carbon chain (palmitate) is formed, the saturated acyl-enzyme complex activates the thioesterase, resulting in the release of palmitate molecule from the enzyme complex. The two -SH sites are now free, allowing another cycle of palmitate synthesis to be initiated.
|
The synthesis of one molecule of palmitate requires eight molecules of AcCoA, 7 ATP, and 14 NADH according to the formula:
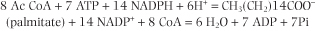
|
| In common with the acetyl CoA carboxylase system, fatty acid synthase activity is also regulated, by substrate flux (the presence of phosphorylated sugars) via an allosteric effect, and by induction and repression of the enzyme.
|
| Alterations in total enzyme protein are effected by the nutritional state of the individual; consequently, this is the main factor controlling the rate of lipogenesis. Rates of fatty acid synthesis are greatest when an individual follows a high-carbohydrate/low-fat diet, and are low during fasting/starvation or when eating high-fat diet. Situations in which there are high circulating concentrations of fatty acids lead to marked inhibition of lipogenesis.
|
|