Central to the recognition of the SRE, and to the binding of the receptor to it, is the presence of the so-called zinc finger region in the DNA-binding domain of the receptor molecule. Zinc fingers consist of a peptide loop with a zinc atom at the core of the loop. In the typical zinc finger, each loop comprises two cysteine and two histidine residues in highly conserved positions relative to each other, separated by a fixed number of intervening amino acids . The consensus sequence for a typical zinc finger is: . The consensus sequence for a typical zinc finger is:
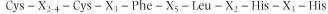 where X represents any intervening amino acid (Fig. 33.5).
where X represents any intervening amino acid (Fig. 33.5).
|
| Figure 33.4 Regulation of gene transcription by glucocorticoids. Steroids bind to receptor molecules that, in turn, bind to an enhancer, the action of which stimulates promoter function. GRE, glucocorticoid response element. |
| page 464 |  | | page 465 |
| This structure holds the zinc atom at the core of a tetrahedron and allows the receptor molecule to sit on the surface of the DNA double helix and interact with a response element, thus enhancing the efficiency of, and conferring specificity to, the promoter. Zinc finger motifs are generally organized as a series of tandem repeat fingers; the number of repeats varies in different transcription factors.
|
The precise structure of the steroid receptor zinc finger differs from the consensus shown above. Steroid receptor zinc fingers have a simpler structure (Fig. 33.5):
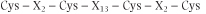
|
| This type of finger recognizes and binds to short palindromic DNA sequences. In the case of steroid receptors, two fingers are found side by side, one binding DNA and the other allowing the receptor to bind to a second steroid receptor molecule of the same type, forming a homodimer.
|
|