Ethanol oxidation reaction by alcohol dehydrogenase (ADH) (EC 1.1.1.1) enzyme in catalytic domain with nicotinamide adenine dinucleotide NAD+ oxidized form NAD+ and zinc Zn2+ ion coordinated alcohol group and His51 hydrogen bond linked water molecule.
H3C-CH2-OH+NAD++H2O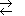 H3C-HC=O+NADH+H3O+ ; E°= -0.113 V;
H3C-HC=O+NADH+H3O+ ; E°= -0.113 V;
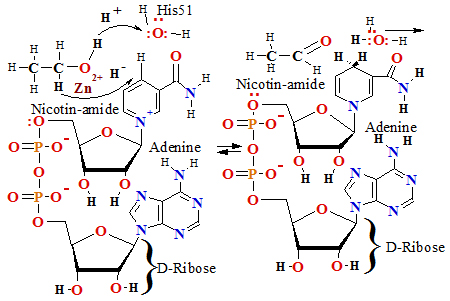
hydride H- ion tuneling from reduced form substrate ethanol H3C-CH2-OH in one attempt to oxidised form NAD+froming reduced form of NADH
in second instant process
jump of proton H+ to water molecule H2O coordinated by zinc Zn2+ ion to form hydroxonium ion H3O+.
ADH during alcohol oxidation in water medium driven by catalytic zinc Zn2+ site moiety coordinated alcohol group -CH2-O-H as acid dissociates proton =>H+ which jump via two amino acids Brønsted basis like as relay assistants Ser-48, His-51, to H2O water molecule forming hydroxonium ion H3O+:
Back to the ADH tutorial

