Back to the tutorial
.....-His96-His94-Zn-His119--Wat-263-.....
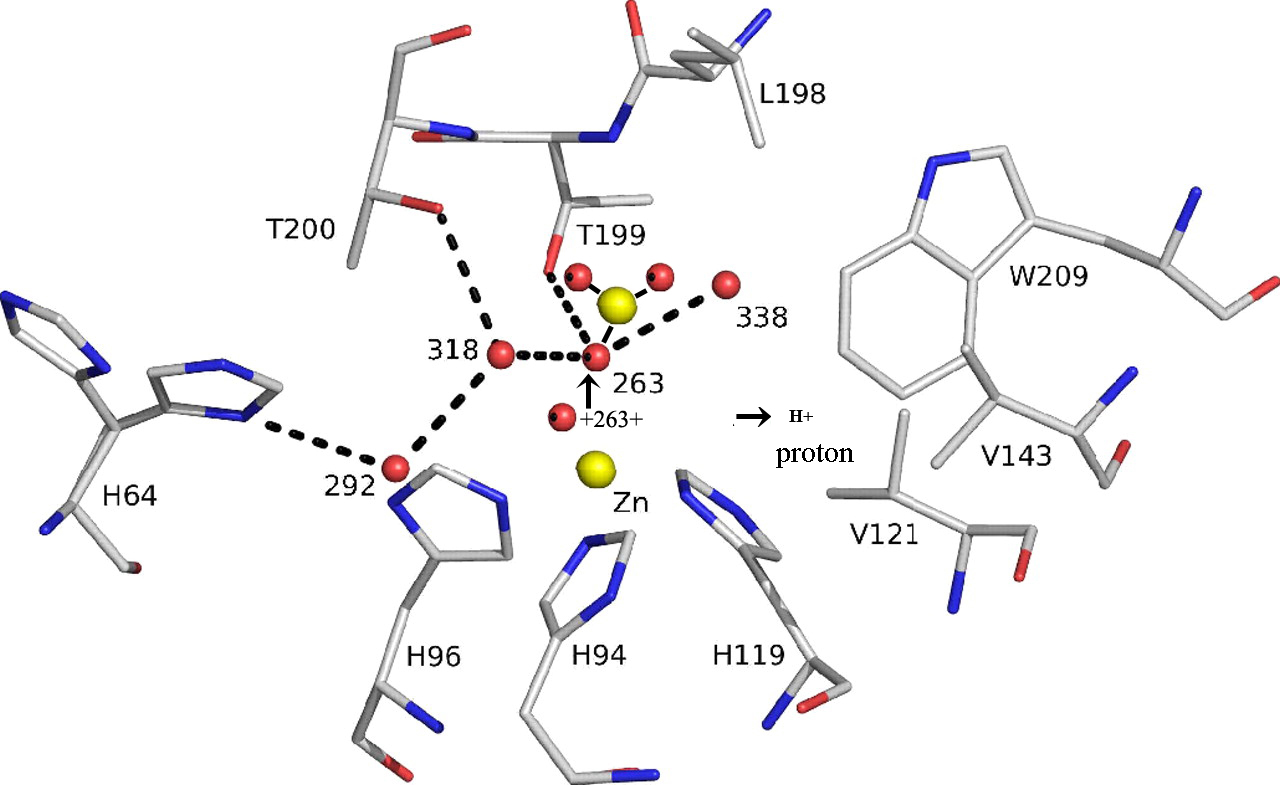
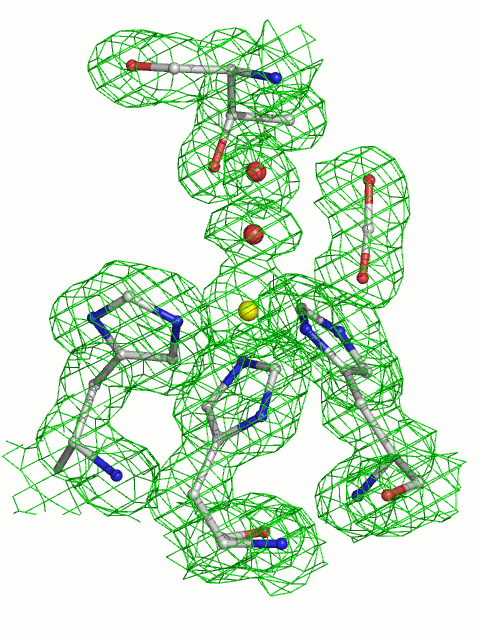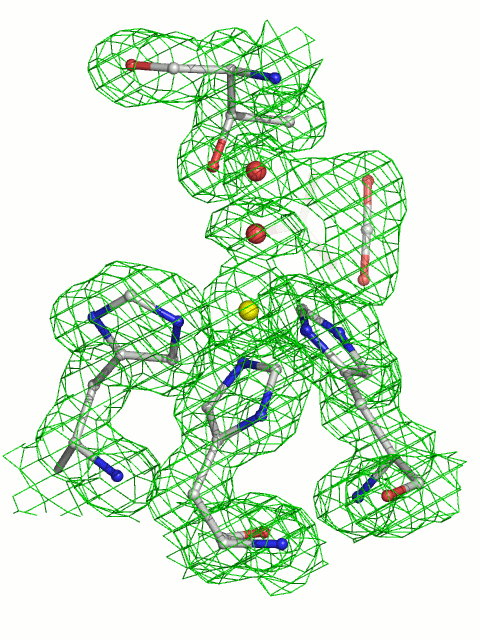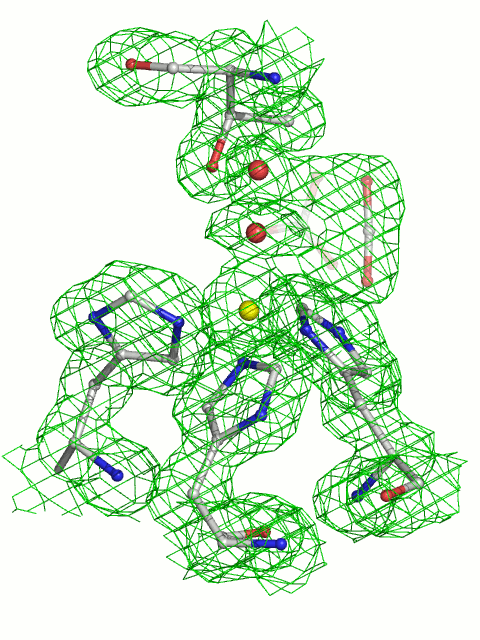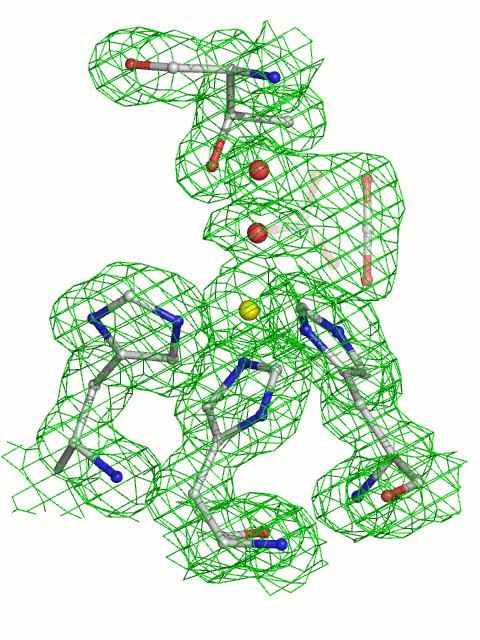
The active site of HCAII. The zinc ion is tetrahedrally coordinated by 3 histidines His-94, His-96, His-119 and catalytic water Wat-263. The deep water Wat-338 sits in a hydrophobic pocket lined by Leu-198, Trp-209, Val-143, Val-121 at the bottom of the active site. Wat-318 is in a hydrophilic environment toward the mouth of the active site cone. The proton shuttle His-64, shown in both “in+H+” and “out (deprotonated)” positions, is linked via Wat-292, Wat-318 to the catalytic waterWat-263. Hydrogen bonds are depicted as dotted lines, and waters are labeled with numbers only. Numbering is according to PDB code 2CBA.
Thr-199, a key residue of the second coordination sphere, is important for enzyme activity; together with Thr-200, it is involved in a finely tuned network of hydrogen bonds leading toward the solvent-exposed His-64, which is located at the entrance of the active-site channel. Thr-199 forms a hydrogen bond to the zinc-bound water/hydroxide Wat-263, thereby orienting the 2 lone hydroxide electron pairs toward the 2 neighboring water molecules (Wat-318 and Wat-338) that reside on potential substrate-binding sites. Although both positions are suitable for a nucleophilic attack of the zinc-bound hydroxide ion, their environments differ substantially.
Wat-318 is located in a hydrophilic environment on the way out of the active-site cone, whereas the “deep water” Wat-338 is located in a hydrophobic pocket that is lined by the following side chains: Leu-198, Trp-209, Val-143, Val-121 (Fig. 1). A wealth of indirect evidence indicates that the deep water Wat-338 position serves as the primary substrate-binding site (1–12), although the atomic details of enzyme and substrate–product interaction have remained elusive until now. The generally accepted catalytic mechanism of carbonic anhydrase is described by a 3-step kinetic scheme: (I) a ZnOH- moiety catalyzes the interconversion of CO2 to HCO3-, leaving a water molecule as the fourth zinc ligand (Eq. 1); (II) a proton is then transferred from the zinc-bound water to the imidazole ring of His-64 (Eq. 2); and (III) this proton then leaves His-64 for the surrounding water H2O (Eq. 3).
His64-E-Zn2+-OH-+CO2+H2O<=>His64-E-Zn2+-OHCO2-+H2O(1a)
His64-E-Zn2+-OHCO2-+H2O<=>His64-E-Zn2+-H2O+HCO3- (1b)
His64-E-Zn2+-H2O<=>H+-His64-E-Zn2+-OH-(2);
H+-His64-E-Zn2+-OH-+H2O<=>His64-E-Zn2+-OH-+H3O+(3)
The pKa values for both the zinc-bound water and the proton shuttle (His-64) are close to 7.