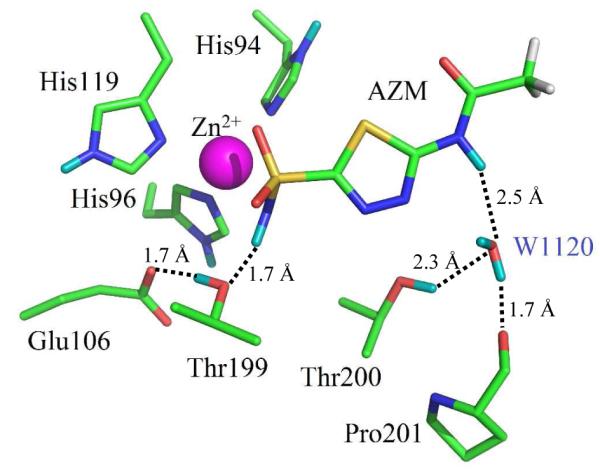
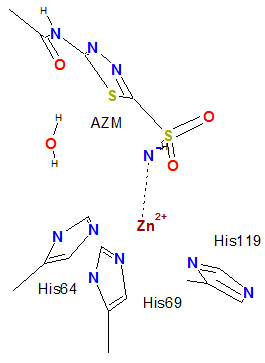
Zn Coordinative Bonds fold in tertiary structure polypeptide chain conecting residues His96,His94,His119 and acetazolamide AZM nitrogen AZM-N after dissociation of H+ in CA active site pocket 4G0C .
Back to the tutorial
.....-His96-His94-Zn-His119--Wat-+263+-.....
On



