The Power of Protons
An Ongoing Attempt to Simplify the Understanding of Calculations involving pH
(Andrew Pearson and Robert Lancashire, University of the West Indies,
Mona Campus, Jamaica.)
Contents:
- Introduction to protons in biochemistry
- Introduction to logarithms
- Proton concentrations and the pH scale
- The Henderson_Hasselbalch Equation
- Using a pH meter
- Some common acid_base titrations
Introduction to protons in biochemistry
Protons are important in biochemistry for many reasons including:
- they carry a positive electrical charge
- they can bind reversibly to many molecules
- most biological fluids contain them
The binding of a proton to an acceptor changes the electrical
charge on the acceptor, and this often changes the biochemical properties of the molecule.
For example:
- it can render the acceptor molecule able to cross lipid bilayers
- it can render the acceptor molecule unable to cross lipid bilayers
- it can render the acceptor molecule able to engage in electrostatic interactions
pK
The willingness of a particular acceptor to bind a proton is an intrinsic property of
the acceptor, governed largely by the behaviour of the electrons in that part of the
acceptor molecule close to the proton binding site. This is called the pK of the group.
pH
It is possible to know how many potential acceptor groups are protonated once the
proton_binding affinity (pK) of the acceptor and the number of available
protons is known. This latter is described in terms of pH.
Small numbers
By international convention the standard number of molecules that is used by
biochemists and chemists is Avogadro's number (6.022 x 1023);
this is called a mole of the molecules.
When a solution is made up containing 1 mole of solute in 1 litre (1 dm_3)
of solvent the concentration of the solute is said to be 1 molar or 1 mol dm_3
(in out_dated notation: 1 M), and this is the standard unit of concentration.
The concentrations of most biochemicals in vivo range between about 10_2
mol dm_3 and 10_12 mol dm_3.
The concentration of protons in a neutral (neither acidic nor basic) aqueous solution
is 10_7 mol dm_3.
Manipulating such small numbers is facilitated by using logarithms.
return to start
Introduction to logarithms
The principle of logarithms is to express a number as another number
with an index or exponent:
actual number = another numberindex, or exponent
e.g. 100 = 102
16 = 24
The "another number" mentioned above is called the "base" of the
logarithm; the most common base numbers used are 10, 2 and a value 'e' ( the base for the
so_called natural logarithms).
For most biochemistry calculations, logarithms to the base 10 are used.
In the days before electronic calculators were readily available to school children
(not so long ago!) logarithm and antilogarithm
tables were easily available, and we had to have a set handy for maths, physics and
chemistry classes and homework.
The following table, in conjunction with the logarithm
table, will require little explanation:
| Number |
Scientific Notation |
Log10 No. |
| 1 |
1 x 100 |
0.0000 |
| 6 |
6 x 100 |
0.7782 |
| 10 |
1 x 101 |
1.0000 |
| 20 |
2 x 101 |
1.3010 |
| 300 |
3 x 102 |
2.4771 |
| 4,000 |
4 x 103 |
3.6021 |
| 5,000,000 |
5 x 106 |
6.6990 |
The log(arithm)10 of the number has two distinct parts separated by a full
stop called "point", which is commonly mistaken for, but is not a simple
decimal point: the integer to the left of the point is called the characteristic
and corresponds to the power to which the multiplier 10 must be raised; the number to the
right of the point is called the mantissa and is determined either by
calculation or by looking up in the tables . The
mantissa is always positive, whereas the characteristic is negative for numbers
that are less than 1.0.
The log & antilog tables shown are four figure tables, and these are accurate
enough for most applications. For more exacting applications, tables are available with
which greater accuracy is possible.
To find the log10 of 45.67 we first inspect the number and determine the
value of the characteristic by converting the number to scientific notation : 4.567 x 101.
The characteristic is thus 1.
We now look up 4567 in the log tables as follows:
the first two digits are to be found in the first column of the table. If the number that
we were looking for were 4500 the mantissa would have been: .6532 (always include the
point in the mantissa).
The mantissa for 4560 is .6590, and the one we really want is .6597 _ having
added the number from the appropriate column in the right hand set of columns to the
mantissa of the first three digits.
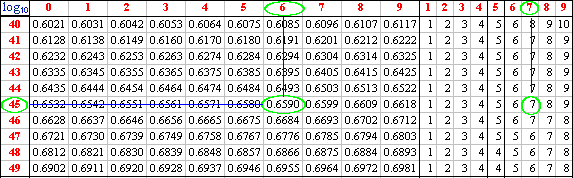
Log10 45.67 = 1.6597
Practice looking up the log10 for a number and
type your answer in the box alongside.
Log10 of a number between 1.0 and zero.
A complication arises for numbers that are less than 1.0, which when expressed in
scientific notation have 10 raised to a negative power such as 0.005 = 5 x 10_3.
The characteristic of the log of this number is called bar three, and
the whole log is called:
"bar three point six nine nine zero"
'Bar three point ...........' is not exactly the same as 'minus three point
...........', and calculations involving pH become very difficult if this is not clearly
understood: much confusion in the correct use of logarthims arises over the difference.
It is only the characteristic that can be negative; the mantissa is always
positive.
This confusion is compounded by the facts that:
- electronic calculators and standard keyboards cannot show bar numbers
- because of the ubiquity of electronic calculators, any formal treatment of logarithms
has dropped out of many high school mathematics syllabuses _ perhaps somebody
should think again!
Since is it not currently possible to use a standard keyboard to enter a bar number, we
will follow the current convention for displaying the characteristic for negative powers
of ten by using the minus sign - .
Thus log10 0.004545 = -3.6585.
Practice looking up the log10 for a number less
than 1 and type your answer in the box alongside.
Now you should be able to reverse the process and use the table to find the number for
which the log10 is 2.8388, however it is slightly easier to look up the
mantissa in the antilog tables, the characteristic merely tells you where to put the
decimal point.
Where a bar characteristic is involved, as with some pH calculations, some
understanding is required. For assistance with examples of the latter we recommend that
you continue with this document up to the end of the next section on the pH scale; or you
can jump immediately to some pH exercises.
These days scientists and their students expecting to have to manipulate logarithms arm
themselves with suitable pocket calculators, but here we find another difficulty: not all
such devices are easy to use or interpret. We strongly urge that you take time here to
practice 'logging' and 'antilogging' numbers with your own calculator, checking the
outputs against the logarithm and tables.
If you are unable to reconcile results, you are advised to:
- either find (if possible!), read and understand (if possible!) the instructional
'manual' that came with your calculator,
- and/or seek direct, expert, human advice,
- or get a better calculator!
return to start
Proton concentrations and the pH scale
Many biochemicals are Br¸nsted_Lowry acids or bases: they can donate
protons if acidic and accept protons if basic such as the carboxylate and amino groups on
the amino acid alanine respectively.
The aqueous environment of most biochemical reactions can exchange protons with such
groups. The proton exchange reaction is governed, as are others, by the concentrations of
the reactants, their willingness to participate and the temperature of the system (which
is really an indication of the speed of the reactants).
Of the willingness to participate (pK in reactions involving protons), strong acids are
more willing to donate their protons than are weak acids: by definition the protons of a
strong acid are always fully dissociated in aqueous solutions, whereas the protons of a
weak acid show a greater affinity for their conjugate base.
We encourage you to look at the accompanying (free) Windows
Titrations program.
Water is both an acid and a base: H2O + H2O = H3O+
+ OH_H3O+ is the hydronium ion, and is a
simple form in which protons can be thought to occur in aqueous solutions. For the sake of
simplicity we shall assume the presence of the H2O molecule associated with the
proton and only write H+. The reaction can thus be written:
H2O = H+ + OH_
To what extent does this dissociation of water molecules (protolysis)
occur? If we could fill a bucket with pure H2O and allowed it to dissociate
(for an infinite length of time) we could then measure the concentrations of the protons
or hydroxyl ions present and determine the acid dissociation constant Ka: Ka
= ([H+] + [OH_]) /[H2O]
(Note that the equilibrium constant is actually a ratio of the activities
of the reactants, and activities are equal to concentrations only in solutions that are ideal
, i.e. which obey Raoult's Law. In practice, provided that we are dealing with
dilute aqueous solutions, we assume that the concentrations of the solutes are the same as
their activities because the former are easier to determine than the latter.)
At 25oC the Ka = 1.8 x 10_16 : there is so
little dissociation that it is reasonable to say that [H2O] is overwhealming
and constant.
We can re_arrange the above equation and introduce a new term Kw
the ion product of water:
Kw = Ka[H2O] = [H+] x [OH_]
= 10_14 mol dm_3 at 25oC
Now in pure water since [H+] must equal [OH_] and
multiplied together they = 10_14mol dm_3,
therefore [H+] = [OH_] = 10_7mol dm_3.
10_7 x 10_7 = 10_7+(_7)
= 10_14
To demonstrate the manipulation of indices and logs10:
| |
10_7 x 10_7 = 10_14 |
_7 + (_7) = _14 |
| therefore |
10_7 = 10_14 / 10_7 |
_7 = _14 _(_7) |
| therefore |
10_7 = 10_14 _(_7) |
_7 = _14 _(_7) |
| therefore |
10_7 = 10_14 +7 |
_7 = _14 +7 |
S¸rensen had the idea of eliminating the minus signs from the log10 of [H+]
and invented the pH scale.
The view that S¸rensen's idea was misguided has been
expressed.
Now in pure water [H+] = [OH_] = 10_7
mol dm_3
therefore the log10 [H+] of pure water = _7
therefore the pH of pure water = 7
If we increase the [H+] of an aqueous solution 100_fold, from 10_7
mol dm_3 to 10_5 mol dm_3,
the pH value changes from 7 to 5.
So the more acid the solution, the lower the pH value;
conversely the pH value rises as the solution becomes more
alkaline.
Try some calculations
- involving negative log10 characteristics
- demonstrating the need for an alternative to the use of a simple negative sign as prefix
- and for consolidation of your understanding of pH, log10 and [H+].
return to start
The Henderson_Hasselbalch Equation
As stated earlier, it is possible to determine the degree of protonation of an acidic
or basic group in an aqueous solution once we know the pK of the group and the pH of the
solution. It is often important to know this because the degree of protonation of a group
will determine its electrical charge and may influence its reactivity.
The pK of such a group is defined as the pH at which the group is exactly 50%
protonated, and is named by analogy to pH: it is _log10K (K =
equilibrium constant).
Dissociation of Acids in Water
HA = H+ + A_ Ka = [H+][A_]
/[HA]In aqueous solution: HA + H2O= H3O+
+ A_ Keq = [H3O+][A_]
/[HA][H2O] = Ka /[H2O]
If we now repeat the earlier assumptions about the constancy and overwhealming
concentration
of H2O, and the equivalence of H3O+ and
H+, we can write: Ka = [H+][A_]/[HA]which is what we had four lines higher. This shows that the
dissociation constant of an acid has the same value as its equilibrium constant in water.
This can be written: [H+] = Ka x [HA] / [A_]Taking logs log10[H+]
= log10Ka + log10([HA] /
[A_])so: _log10[H+] = _log10K
a _ log10([HA] / [A_])and
pH = pKa _ log10([HA] /
[A_])
Note that since _ log10a /
b = + log10b / a, it could be written: pH
= pKa + log10([A_] /
[HA])
Both are called the Henderson_Hasselbalch Equation: beware of the
last term in the equation since many mistakes are made with the sign and decision of which
concentration is to be divided by the other.
Consider the effect of putting pH = pKa
Therefore pKa = pKa _ log10
([HA] / [A_])
Therefore 0 = _ log10([HA] /
[A_])Since antilog 0.0000 is 1.0000, then (remembering the negative
sign!)
1 = [A_] /
[HA]Therefore: [HA] = [A_]
This means that the acid is exactly half dissociated.
Consider the effect of putting pH = pKa + 1. This involves a
ten_fold change in [H+]:
[H+] is now ten times less than the [H+] at 50% dissociation.
pKa + 1 = pKa _ log10([HA]
/ [A_])
Therefore 1 = _ log10([HA] /
[A_])
Since antilog 1 is 10, then (remembering the negative sign!)
10 = [A_] / [HA]
Therefore: 10[HA] = [A_]
This means that if we had eleven molecules of acid, only one would be protonated at
that [H+], and ten would be unprotonated.
Let us consider the amino acid alanine which has an acidic carboxyl
group and a basic amino group. 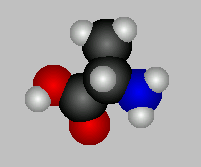
When the [H+] is high, say pH = 1, both groups will be protonated and alanine
will have a net charge of +1.
When pH = pK of the carboxyl group at 2.34, protons will have dissociated from half of
the alanine carboxyls present and the net charge will be + 0.5, since the amino group will
still be fully protonated and thus carry a positive charge but the unprotonated half of
the carboxyl groups present will carry a negative charge.
Further reducing the [H+] of the solution, by adding NaOH for example, will
drag the rest of the protons from the alanine carboxyls present and the net charge will be
zero. The pK of the amino group of alanine is 9.69 so that when the pH of the solution
reaches this value, the net charge will be _0.5.
return to start
Using a pH meter
return to start
Some common acid_base titrations
Free Titrations Windows program.
Created and maintained by Andrew Pearson and Robert Lancashire,
University of the West Indies, Mona Campus, Jamaica.
Comments to authors at Andrew Pearson and/or Robert Lancashire.
Created Monday Apr 15th 1996. Last modified 13th Septmeber 1999.
URL: http://wwwchem.uwimona.edu.jm:1104/courses/pH/phlogs.html


