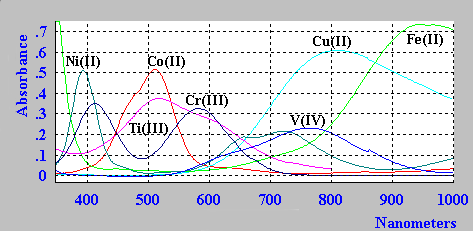
The spectra of the aqua ions for some first row transition metal ions are shown below.

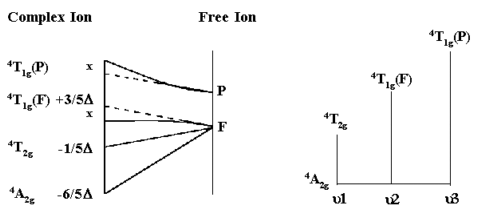
For d3,d8 octahedral and d2, d7 tetrahedral complexes, the above diagrams can be used to interpret the observed electronic absorption spectra.
Take for example the Cr3+ aquo ion [Cr(H2O)6]3+.
From the simplified Orgel diagram, three absorptions are expected. In practice, the
spectrum is found to contain 3 bands which occur at 17,000 cm-1, 24,000 cm-1
and 37,000 cm-1. Of which only two are shown above.
u1 corresponds exactly to D (Delta) and since the lowest band is found at 17,000 cm-1 then this enables us to measure D directly from the spectrum.
The next band is found at 24,000 cm-1 and this can be equated to:
u2=9/5D - x where x is the configuration
interaction between the T(F) state and the T(P) state of the same symmetry.
Since D is 17,000 and u2 is observed at 24,000 then x
must be 6,600 cm-1.
The last band is seen at 37,000 cm-1 and here
u3=6/5D + 15B + x where B is one of the RACAH
parameters.
Solving for B gives a value of B=667cm-1.
For the free Cr3+ ion, B is ~1030 cm-1 so that in the complex this
term is reduced by ~2/3 of the free ion value.
A large reduction in B indicates a strong Nephelauxetic Effect. The Nephelauxetic Series is given by:
F- > H2O > urea > NH3 > en ~ C2O42- > NCS- > Cl- ~ CN- > Br- > S2- ~ I-
Ionic ligands such as F- give a small reduction in B,
while covalently bonded ligands such as I- give a large reduction of B.
 Return to Chemistry, UWI-Mona, Home Page
Return to Chemistry, UWI-Mona, Home Page
Created 20th Sept 1995. Last modified 20th January-99, rjl