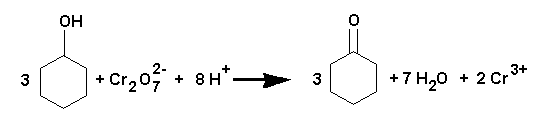
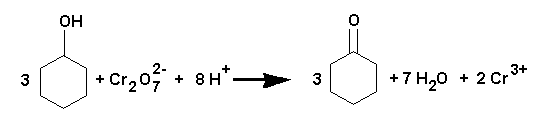
This preparation shows that a ketone can be prepared by the oxidation of a secondary alcohol. In a similar way, an aldehyde can be prepared from a primary alcohol, but since aldehydes are easily oxidised further to carboxylic acids, they must be distilled off from the reaction mixture as formed.
RCH2OH -> RCHO -> RCO2H
(1 alcohol) (aldehyde) (acid)
Dissolve sodium dichromate dihydrate (12.5 g) in water (60 mL) in a 100 mL beaker and add slowly and carefully, with continuous stirring (NOTE) with a glass rod, concentrated sulphuric acid (11 g, 6 mL). Allow the mixture to cool. Place cyclohexanol (6 g, density 0.96 g cm-3) in a 100 mL conical flask and add the dichromate solution to it in one portion, with swirling to ensure thorough mixing. When the temperature rises to 55° , cool the flask in cold water; sufficient external cooling should be applied to keep the temperature between 55° and 60° . When the mixture no longer heats up, leave to stand with occasional swirling for 1 hour.
Pour the mixture into a 250 mL round bottom flask, add water (60 mL), and fit up a distillation apparatus (but with a stopper instead of a thermometer and a 100 mL graduated cylinder to collect the distillate). Distil the mixture over a Bunsen until about 30 mL of distillate (two layers) has been collected. Saturate with salt (about 7 g) and separate the layer of cyclohexanone in a separatory funnel. Extract the aqueous layer twice with ethyl acetate (10 mL). Combine the ethyl acetate extracts with the cyclohexanone layer. Dry with anhydrous magnesium sulphate (5 min) and filter into a dry 50 mL RB flask. Distil off the ethyl acetate, b.p. 77° , using a distillation apparatus set up on a steam bath. Distil the crude cyclohexanone using a Bunsen and wire gauze (b.p. 153° - 156° ). Collect the fraction distilling above 100° . Before discarding your dark green chromium (III) sulphate solution down the sink add 2 mL ethanol and swirl.
Inspect the i.r. spectra of cyclohexanol and cyclohexanone. Record on your work sheet the position of the band and name the functional group which disappears during the oxidation reaction. Also, record the position of the band and name the functional group which appears during the oxidation reactions.
IR of thin film of cyclohexanol
IR of thin film of cyclohexanone
Carry out the tests for aldehydes and ketones (Jones and DNP tests, pp 33 - 34) with the samples provided. Record your results in tabular form.
NOTE: Do not stir the mixture with your thermometer!
1. Explain why ethanol is added to the residue in the distillation flask?
2. Why is this important?
 Return
to Chemistry, UWI-Mona, Home Page
Return
to Chemistry, UWI-Mona, Home Page