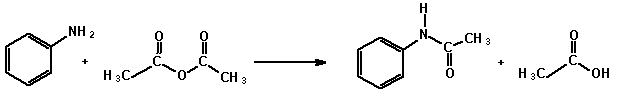
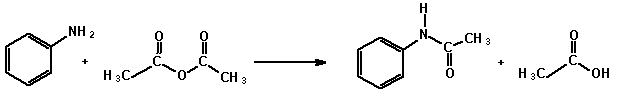
To 60 mL water in a 100 mL Erlenmeyer flask add 2.5 mL concentrated hydrochloric acid with mixing. In the fume hood add 2.5 mL aniline (density 1.02 g cm-3) and swirl the mixture. If the solution is coloured, add a small amount of decolourising charcoal, swirl the flask for about one minute, and filter off the carbon. In a separate container dissolve 4 g sodium acetate in 10 mL water. Warm the anilinium chloride solution to 50 on a water bath and add 3 mL acetic anhydride (density 1.08 g cm-3). Swirl to effect dissolution and add the aqueous sodium acetate quickly. Swirl the flask a couple of times and set it in an ice-bath for 20 min. Filter with suction the crystals of the amide formed and wash with a small amount of ice-cold water. Dry the material between filter papers and submit your sample for assessment.
Inspect the i.r. spectra of the starting material and product and record in your lab book the position of the band which appears during the acetylation (see Appendix 2). Relate this to the reaction occurring.
Carry out the nitrous acid test (p 30) on the 1 , 2 and 3 amines (aliphatic and aromatic) provided. Record your results in tabular form.
 Return to Chemistry, UWI-Mona, Home Page
Return to Chemistry, UWI-Mona, Home Page
Mar-97, pbr, rjl