Since the discovery in 1948 that Co was an essential part of Vitamin B12, the isolation, structural determination and synthesis of the vitamin have been accomplished and the final pieces of it's biosynthetic pathway are nearly completed. This is a remarkable achievement considering that per cm3 of blood there is only about 10-10 g of vitamin B12 found!
However, the role of the vitamin is not yet fully understood, although it is known that it is essential in preventing the disease pernicious anaemia.
Three biologically active cobalamins can be detected in the serum and tissues of man and higher animals, these are: adenosylcobalamin, AdoCbl; hydroxocobalamin, OH-Cbl; and methyl-cobalamin, MeCbl. Cyanocobalamin, CN-Cbl has been detected in chromatograms of the blood of smokers, where it is believed to be formed from HCN from cigarettes reacting with OH-Cbl. It has no physiological role (apart from this unexpected detoxification of cyanide) although it has been used for treating pernicious anaemia because like OH-Cbl, it can be converted in vivo into Ado-Cbl and MeCbl.
Pernicious anaemia is largely a disease affecting people over the age of 40 and is
almost unknown in negroes. In the UK, it occurs with a frequency of 1 in 1000. The
consequence of depriving people of essential cobalamins is debilitation which if continued
can end in death.
The model compound to be prepared in this experiment contains an ethyl-cobalt bond. The
cobaloximes have been used as models due to their ease of preparation, purification and
characterisation.
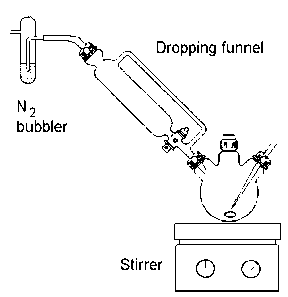
A 250 cm3 three necked flask is equipped with a pressure equalising dropping funnel which in turn is attached to a gas outlet bubbler. The central neck is stoppered and the third neck is equipped with an inlet to bubble nitrogen gas through the reactants. This enables the system to be deoxygenated and allows the later stages of the experiment to be conducted with strict exclusion of oxygen. A mixture of methanol (100 cm3) and pyridine (0.8 g, 11mmol) is magnetically stirred under nitrogen flow for several minutes.

Carefully remove the stopper briefly (increase the gas flow momentarily) then
dimethylglyoxime (2.32 g, 20 mmol) is added, followed by finely powdered cobalt(II)
chloride hexahydrate (2.37 g, 10 mmol). Not all the reactants will dissolve but the deep
brown colouration due to the Co(II) complex should appear. Sodium hydroxide (1.6 g, 40
mmol) dissolved in the minimum amount of water (about 5 cm3) is added via the
dropping funnel. The intense blue-black colour of the cobalt(I) intermediate should
develop over the next 2-4 minutes. If it does not then air has attacked the cobalt(I)
intermediate and the experiment should be repeated (see the demonstrator first).
Next, a solution of iodoethane (0.78 g, 5 mmol) dissolved in a little methanol (about 5 cm3)
is added, again via the dropping funnel. The organocobaloxime forms immediately as
evidenced by the blue suspension turning brown. The methanol solvent is removed by rotary
evaporation at about 50 óC(higher temperatures may lead to decomposition). The pasty
mass is stirred with a slush of ice and water (about 200 cm3). This oxygenates
and solubilises the unwanted by-products but does not significantly decrease the yield of
product.
The orange brown cobalt complex is filtered on a sintered glass funnel and washed with
100cm3 of ice-cold water. Dry in a dessicator in the dark (the product is
somewhat light sensitive) and record the yield.
The proton NMR of the complex recorded in MeOD shows a very strong peak around 2 ppm.
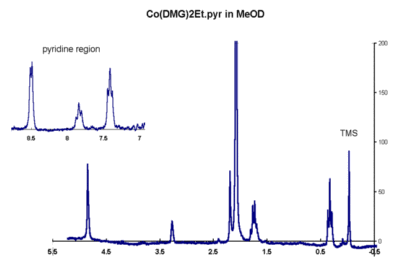 NMR spectrum in JCAMP-DX format
NMR spectrum in JCAMP-DX format
Account for this and the appearance of the other peaks in the spectrum.
An IR spectrum is available as well. Identify as many peaks as you can.
1) Write brief notes on the suspected role of vitamin B12 in biological systems.
B. T. Golding, Chem. in Britain, 26, 1990, 950.
Hill et al., J. Chem. Soc (A), 1969, 554.
R.H. Abeles and D. Dolphin, Accounts Chem. Res., 9, 1976, 114.
Comprehensive Organometallic Chemistry, Ed G. Wilkinson, Pergamon Press, 1984.vol 5.
return to list of C31L experiments
 Return to Chemistry, UWI-Mona, Home Page
Return to Chemistry, UWI-Mona, Home Page
Nov-96, rjl