| Enzymes are inhibited in different ways
|
| Among numerous substances affecting metabolic processes, enzyme inhibitors are particularly important. Many drugs, either naturally occurring or synthetic, act as enzyme inhibitors. Metabolites of these compounds may also inhibit enzyme activity. Most enzyme inhibitors act reversibly, but there are also irreversible inhibitors that permanently modify the target enzyme. Using Lineweaver-Burk plots, it is possible to distinguish three forms of reversible inhibition: competitive, uncompetitive, and noncompetitive inhibition.
|
| Competitive inhibitors cause an apparent increase in Km, without changing Vmax
|
| METHANOL POISONING CAN BE TREATED BY ETHANOL ADMINISTRATION |
| A 46-year-old male presented to the emergency room 7h after consuming a large quantity of bootleg alcohol. He could not see clearly and complained of abdominal and back pain. Laboratory results indicated severe metabolic acidosis, a serum osmolality of 465 mmol/kg (reference range 285-295 mmol/kg), and serum methanol level of 4.93 g/L. By aggressive treatment, including an ethanol drip, bicarbonate, and hemodialysis, he survived and regained his eyesight. |
Comment. Methanol poisoning is uncommon but extremely hazardous. Ethylene glycol poisoning is more common and exhibits similar clinical characteristics. The most important initial symptom of methanol poisoning is visual disturbance. Laboratory evidence of methanol poisoning includes severe metabolic acidosis and increased plasma solute concentration. Methanol is slowly metabolized to formaldehyde , which is then rapidly metabolized to formate by alcohol dehydrogenase. Formate accumulates during methanol intoxication and is responsible for the metabolic acidosis in the early stage of intoxication. In later stages lactate may also accumulate as a result of formate inhibition of respiration. Ethanol is metabolized by alcohol dehydrogenase, which binds ethanol with much higher affinity than either methanol or ethylene glycol. Ethanol is therefore a useful agent to inhibit competitively the metabolism of methanol and ethylene glycol to toxic metabolites. Early treatment with ethanol, together with alkali to combat acidosis and hemodialysis to remove methanol and its toxic metabolites, yields a good prognosis. , which is then rapidly metabolized to formate by alcohol dehydrogenase. Formate accumulates during methanol intoxication and is responsible for the metabolic acidosis in the early stage of intoxication. In later stages lactate may also accumulate as a result of formate inhibition of respiration. Ethanol is metabolized by alcohol dehydrogenase, which binds ethanol with much higher affinity than either methanol or ethylene glycol. Ethanol is therefore a useful agent to inhibit competitively the metabolism of methanol and ethylene glycol to toxic metabolites. Early treatment with ethanol, together with alkali to combat acidosis and hemodialysis to remove methanol and its toxic metabolites, yields a good prognosis. |
An enzyme can be inhibited competitively by substances that are similar in chemical structure to the substrate (Fig. 5.6). These compounds compete with substrate for the active site of the enzyme causing an apparent increase in Km, but no change in Vmax. The inhibition is not the result of an effect on enzyme activity, but on substrate access to the active site. The kinetic scheme for competitive inhibition, is:
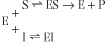
|
| The inhibition constant (Ki) is the dissociation constant of the enzyme-inhibitor complex (EI). The lower the Ki, the more efficient the inhibition of enzyme activity.
|
| BothKm andc Vmax decrease in uncompetitive inhibition
|
An uncompetitive inhibitor binds only to the enzyme-substrate complex and not to the free enzyme. The equation shows the kinetic scheme for uncompetitive inhibition. In this case, the Ki is the dissociation constant for the enzyme-substrate-inhibitor complex (ESI).
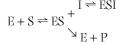
|
| page 57 |  | | page 58 |
| Figure 5.6 Competitive enzyme inhibition. (A) Plot of velocity versus substrate concentration; (B) mechanism of competitive inhibition; (C) Lineweaver-Burk plot in the presence of a competitive inhibitor; and (D) Eadie-Hofstee plot in the presence of a competitive inhibitor. K'm is the apparent Km in presence of inhibitor. |
| Figure 5.7 Structure of penicillin showing the reactive peptide bond in the β-lactam ring and core structure of cephalosporins. Penicillins contain β-lactam ring in conjunction with thiazolidine ring. Cephalosporins are another class of compounds containing the β-lactam ring fused to a six-membered dihydrothiazine ring. Because of their effectiveness and lack of toxicity, β-lactam compounds are widely used antibiotics. Bacteria with β-lactamase, which breaks the β-lactam ring, are resistant to these antibiotics. |
| ENZYME INHIBITION: TRANSITION-STATE INHIBITION AND SUICIDE SUBSTRATE |
| Enzymes catalyze reactions by inducing the transition state of the reaction. It should therefore be possible to construct molecules that bind very tightly to the enzyme by mimicking the transition state of the substrate. Transition states themselves cannot be isolated, because they are not a stable arrangement of atoms, and some bonds are only partially formed or broken. But for some enzymes, analogues can be synthesized that are stable, but still have some of the structural features of the transition state. |
| Penicillin (Fig. 5.7) is a good example of a transition state analog. It inhibits the transpeptidase that crosslinks bacterial cell-wall peptidoglycan strands, the last step in cell-wall synthesis in bacteria. It has a strained 4-membered lactam ring that mimics the transition state of the normal substrate. When penicillin binds to the active site of the enzyme, its lactam ring opens, forming a covalent bond with a serine residue at the active site. Penicillin is a potent irreversible inhibitor of bacterial cell-wall synthesis, making the bacteria osmotically fragile and unable to survive in the body. |
| The inhibitor causes a decrease in Vmax because a fraction of the enzyme-substrate complex is diverted by the inhibitor to the inactive ESI complex. Bound inhibitor also affects the dissociation of substrate, causing an apparent decrease in Km, i.e. an apparent increase in sub-strate affinity.
|
| page 58 |  | | page 59 |
| Vmax decreases in noncompetitive inhibition
|
A noncompetitive inhibitor can bind either to the free enzyme or to the enzyme-substrate complex. Thus, noncompetitive inhibition is more complex than other types of inhibition. The next equation shows the kinetic pattern observed for noncompetitive inhibition.
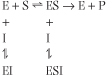
|
| Many drugs and poisons irreversibly inhibit enzymes
|
Prostaglandins are key inflammatory mediators. Their synthesis is initiated by cyclooxygenase-mediated oxidation and cyclization of arachidonate under inflammatory conditions (Chapter 38). Compounds that suppress cyclooxygenase have anti-inflammatory activity. Aspirin (acetylsalicylic acid), one of the most popular drugs, inhibits cyclooxygenase activity by acetylating Ser530, which blocks access of arachidonate to the active site of the enzyme. Other nonsteroidal anti-inflammatory drugs (NSAIDs), such as indomethacin (acetylsalicylic acid), one of the most popular drugs, inhibits cyclooxygenase activity by acetylating Ser530, which blocks access of arachidonate to the active site of the enzyme. Other nonsteroidal anti-inflammatory drugs (NSAIDs), such as indomethacin , inhibit cyclo-oxygenase activity by reversibly blocking the channel for arachidonate in the enzyme. , inhibit cyclo-oxygenase activity by reversibly blocking the channel for arachidonate in the enzyme.
|
Disulfiram (Antabuse®) is a drug used for the treatment of alcoholism. Alcohol is metabolized in two steps to acetic acid . The first enzyme, alcohol dehydrogenase, yields acetaldehyde, which is then converted into acetic acid . The first enzyme, alcohol dehydrogenase, yields acetaldehyde, which is then converted into acetic acid by aldehyde dehydrogenase. The latter enzyme has an active site cysteine residue that is irreversibly modified by disulfiram by aldehyde dehydrogenase. The latter enzyme has an active site cysteine residue that is irreversibly modified by disulfiram , resulting in accumulation of alcohol and acetaldehyde in the blood. People who take disulfiram , resulting in accumulation of alcohol and acetaldehyde in the blood. People who take disulfiram become sick because of the accumulation of acetaldehyde in blood and tissues, leading to alcohol avoidance. become sick because of the accumulation of acetaldehyde in blood and tissues, leading to alcohol avoidance.
|
| Alkylating reagents, such as iodoacetamide (ICH2 - CONH2), irreversibly inhibit the catalytic activity of some enzymes by modifying essential cysteine residue. Heavy metals, such as mercury and lead salts, also inhibit sulfhydryl enzymes. The mercury adducts are often reversible by thiol compounds. Eggs or egg-white are sometimes administered as an antidote for accidental ingestion of heavy metals; the egg white protein, ovalbumin, is rich in sulfhydryl groups, traps the free metal ions and prevents their absorption from the gastrointestinal tract.
|
| In many cases, irreversible inhibitors are used to identify active-site residues involved in enzyme catalysis and to gain insight into the mechanism of enzyme action. By sequencing the protein, it is possible to identify the specific amino acid residue modified by the inhibitor and involved in catalysis.
|
| CATALYTIC MECHANISM OF SERINE PROTEASE |
In enzyme reactions, ionizable amino acids , such as histidine and cysteine, participate in the enzyme-catalyzed reaction. In the serine protease family, a specific serine residue forms the catalytic center, with aid from other amino acids , such as histidine and cysteine, participate in the enzyme-catalyzed reaction. In the serine protease family, a specific serine residue forms the catalytic center, with aid from other amino acids . Ser195, His57, and Asp102 form a 'catalytic triad' in chymotrypsin . Ser195, His57, and Asp102 form a 'catalytic triad' in chymotrypsin (Fig. 5.8). When the proton of the hydroxyl group of Ser195 is hydrogen bonded to His57, the more nucleophilic oxygen atom of ser195 is able to attack the carbonyl carbon atom of the peptide bond in the substrate. The role of the carboxylate group of Asp102 is to stabilize the positively charged form of His57 in the transition state. (Fig. 5.8). When the proton of the hydroxyl group of Ser195 is hydrogen bonded to His57, the more nucleophilic oxygen atom of ser195 is able to attack the carbonyl carbon atom of the peptide bond in the substrate. The role of the carboxylate group of Asp102 is to stabilize the positively charged form of His57 in the transition state. |
Trypsin and elastase, two other digestive enzymes, are similar to chymotrypsin , in many respects. About 40% of the amino acid sequences of these three enzymes are identical, and their three-dimensional structures are very similar. All three enzymes contain a serine-histidine-aspartate-catalytic triad, and are inactivated by the binding of fluorophosphates such as diisopropylfluorophosphate to the serine residue in this triad. , in many respects. About 40% of the amino acid sequences of these three enzymes are identical, and their three-dimensional structures are very similar. All three enzymes contain a serine-histidine-aspartate-catalytic triad, and are inactivated by the binding of fluorophosphates such as diisopropylfluorophosphate to the serine residue in this triad. |
| Figure 5.8 A schematic model of a catalytic triad of serine protease. |
|