| Transport mediated by membrane proteins
|
Transport of larger, polar molecules, such as amino acids or sugars, into a cell requires the involvement of membrane proteins known as transporters, also called porters, permeases, translocases, or carrier proteins. The term 'carrier' is also applied to ionophores, which move passively across the membrane together with the bound ion (Fig. 7.4). Transporters are as specific as are enzymes for their substrates, and work by one of two mechanisms: facilitated diffusion or active transport. Facilitated diffusion catalyzes the movement of a substrate through a membrane down a concentration gradient and does not require energy. In contrast, active transport is a process in which substrates are transported uphill, against their concentration gradient. Active transport must be coupled to an energy-producing reaction (see Fig. 7.3A). or sugars, into a cell requires the involvement of membrane proteins known as transporters, also called porters, permeases, translocases, or carrier proteins. The term 'carrier' is also applied to ionophores, which move passively across the membrane together with the bound ion (Fig. 7.4). Transporters are as specific as are enzymes for their substrates, and work by one of two mechanisms: facilitated diffusion or active transport. Facilitated diffusion catalyzes the movement of a substrate through a membrane down a concentration gradient and does not require energy. In contrast, active transport is a process in which substrates are transported uphill, against their concentration gradient. Active transport must be coupled to an energy-producing reaction (see Fig. 7.3A).
|
| Figure 7.4 Mobile ion carriers and channel-forming ionophores. Ionophores permit net movement of ions only down their electrochemical gradients. |
| Saturability and specificity are important characteristics of the membrane transport systems
|
The rate of facilitated diffusion is generally much greater than that of simple diffusion: transport proteins catalyze the transport process. In contrast to simple diffusion, in which the rate of transport is directly proportional to the substrate concentration, facilitated diffusion is a saturable process, characterized by a maximum transport rate, Tmax (Fig. 7.5). When the concentration of extracellular molecules (transport substrates) becomes very high, the Tmax is achieved by saturation of the transporter proteins with substrate. The kinetics of facilitated diffusion for substrates can be described by the same equations that are used for enzyme catalysis (e.g. Michaelis-Menten and Lineweaver-Burk type equations) (see Chapter 5):
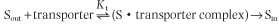
- where Kt is the dissociation constant of substrate (S) and transporter, and Sout is the concentration of transport substrate. Then the transport rate, t, can be calculated as:
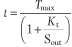 - where the Kt is the concentration that gives the half-maximal transport rate. The Kt for a transporter is conceptually the same as the Km for an enzyme.
|
| The transport process is usually highly specific: each transporter transports only a single species of molecules or structurally related compounds. The red blood cell GLUT-1 transporter has a high affinity for d-glucose, but 10-20 times lower affinity for the related sugars, d-mannose and d-galactose. The enantiomer l-glucose is not transported; its affinity is more than 1000 times less than that of the d-form.
|
| page 82 |  | | page 83 |
| Antibiotics that induce ion permeability |
| Peptide antibiotics act as ionophores and increase the permeability of membranes to specific ions; bactericidal effects of ionophores are attributed to disturbance of the ion transport systems of bacterial membranes. Ionophores permit net movement of ions only down their electrochemical gradients. There are two classes of ionophores: mobile ion carriers (or caged carriers) and channel formers (Fig. 7.4). Valinomycin is a typical example of a mobile ion carrier. It is a cyclic peptide with a lipophilic exterior and ionic interior. It dissolves in the membrane and diffuses between the inner and outer surfaces. K+ binds to the central core of valinomycin, and the complex diffuses across the membrane, releasing the K+ and gradually dissipating the K+ gradient. The carrier type-ionophores, nigericin and monensin, exchange H+ for Na+ and K+, respectively. Ionomycin and A23187 are Ca2+ ionophores. |
| The β-helical gramicidin A molecule, a linear peptide with 15 amino acid residues, forms a pore. The head-to-head dimer of gramicidin A makes a transmembrane channel that allows movement of monovalent cations (H+, Na+, and K+). Alamethicin forms volatage-gated channels developing cation conductance. |
Polyene antibiotics such as amphotericin B and nystatin and nystatin exert their cytotoxic action by rendering the membrane of the target cell permeable to ions and small molecules. Formation of a sterol-polyene complex is essential for the cytotoxic function of these antibiotics, as they display a selective action against organisms in which the membranes contain sterols. Thus they are active against yeasts, a wide variety of fungi, and other eucaryotic cells, but have no effect on bacteria. Because their affinity toward ergosterol, a fungal membrane component, is higher than that for cholesterol, these antibiotics have been used for the treatment of topical infections of fungal origin. exert their cytotoxic action by rendering the membrane of the target cell permeable to ions and small molecules. Formation of a sterol-polyene complex is essential for the cytotoxic function of these antibiotics, as they display a selective action against organisms in which the membranes contain sterols. Thus they are active against yeasts, a wide variety of fungi, and other eucaryotic cells, but have no effect on bacteria. Because their affinity toward ergosterol, a fungal membrane component, is higher than that for cholesterol, these antibiotics have been used for the treatment of topical infections of fungal origin. |
|