| Transduction of energy from reduced coenzymes to high-energy phosphate
|
The oxidation of reduced nucleotides by the electron transport system produces a large amount of free energy. When the oxidation of one mole of NADH is coupled to the reduction of ½ mole of oxygen to form water, the energy produced is theoretically sufficient to synthesize 7 moles of ATP:
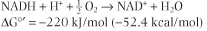
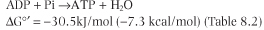
|
| Dividing 220 kJ/mol of ΔG°' available from oxidation of NADH by ΔG°' 30.5 required for synthesis of ATP yields theoretically ∼7 mol ATP/mol NADH. As discussed below, the actual yield is closer to 3 mol ATP/mol NADH oxidized.
|
| page 96 |  | | page 97 |
| Figure 8.3 The structure of redox coenzymes. NAD+ and its reduced form, NADH (nicotinamide adenine dinucleotide), consists of adenine, two ribose units, two phosphates, and nicotinamide; FAD and its reduced form, FADH2 (flavin adenine dinucleotide) consists of riboflavin, two phosphates, ribose and adenine; FMN and FMNH2 consist of riboflavin phosphate. The nicotinamide and riboflavin components of these coenzymes are reversibly oxidized and reduced during electron transfer (redox) reactions. NADH and FADH2 are often called reduced nucleotides or reduced coenzymes. |
| METABOLIC FUNCTION OF ATP REQUIRES MAGNESIUM |
| ATP readily forms a complex with magnesium ion, and it is this complex that is required in all reactions in which ATP participates, including its synthesis. A magnesium deficiency impairs virtually all of metabolism, because ATP can neither be made nor utilized in adequate amounts. |
| The free energy of oxidation of NADH is used via the electron transport system to pump protons into the intermembrane space. The energy produced when these protons re-enter the mitochondrial matrix are used to synthesize ATP. This process is known as oxidative phosphorylation (Fig. 8.4).
|
|