| THE MITOCHONDRIAL ELECTRON TRANSPORT SYSTEM
|
| page 97 |  | | page 98 |
|
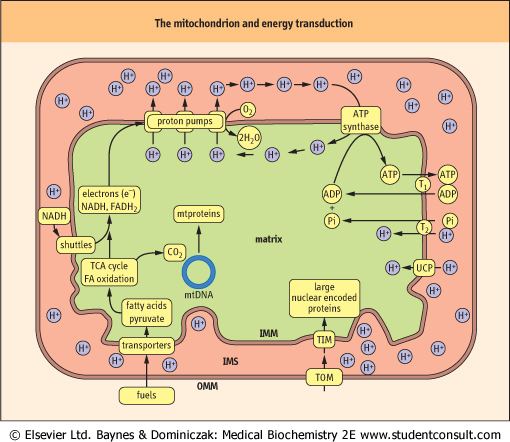
| Figure 8.4 Mitochondrial structure and pathways of energy transduction: the mechanism of oxidative phosphorylation. Major fuels, such as pyruvate and fatty acids (FA), are transported into the matrix where they are oxidized to generate CO2 and the reduced nucleotide coenzymes NADH and FADH2. Oxidation of these nucleotides via the electron transport system reduces oxygen to water and pumps protons by three proton pumps out of the matrix and into the intermembrane space (IMS), creating a pH gradient, which is the major contributor to the membrane potential. It should be noted that protons in the intermembrane space freely diffuse through the outer membrane via the protein porin, so the intermembrane space is roughly equivalent to the cytosol. Although the membrane potential is mostly comprised of the proton gradient it actually consists of several electrochemical gradients and is expressed as a voltage. Controlled influx of protons through ATP synthase powers the synthesis of ATP by ATP synthase. Mitochondrial ATP is then exchanged for cytoplasmic ADP through the ADP-ATP translocase (T1). Phosphate (Pi), which is also required for ATP synthesis is transported by the phosphate translocase (T2). The inner membrane also contains uncoupling proteins (UCP) that may be used to allow the controlled leakage of protons back into the matrix. OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; mtproteins, mitochondrial proteins; mtDNA, mitochondrial DNA; TOM and TIM, protein translocase complexes in outer and inner mitochondrial membrane; TCA, tricarboxylic acid cycle. |
| The entire electron transport system, also known as the electron transport chain or respiratory chain, is located in the
inner mitochondrial membrane (Fig. 8.5). It consists of several large protein complexes and two small, independent components, ubiquinone and cytochrome c. Each step involves a redox reaction where electrons leave components with more negative reduction potentials and go to components with more positive reduction potentials. Electrons are conducted through this system in a defined sequence from reduced nucleotide coenzymes to oxygen, and the free energy changes drive the transport of protons from the matrix into the intermembrane space via the three proton pumps. After each step, the electrons are at a lower energy state.
|
Electrons are funneled into the electron transport chain by several flavoproteins. Of these, there are four major species, including complex I, which contains FMN, and three that contain FAD. These pathways all reduce the small, lipophilic molecule, ubiquinone (Q or coenzyme Q10), at the beginning of the common electron transport pathway, consisting of Q, complex III, cytochrome c, and complex IV.
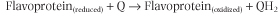
|
| page 98 |  | | page 99 |
| Figure 8.5 A section of the mitochondrial inner membrane with the electron transport system and ATP synthase. I, Complex I; II, Complex II; III, Complex III; IV, Complex IV; V, Complex V or ATP synthase; G, glycerol 3-phosphate dehydrogenase; F, fatty acyl CoA dehydrogenase; Q, ubiquinone; c, cytochrome c; UCP, uncoupling protein. |
| Protons are pumped from the matrix into the intermembrane space by complexes I, III, and IV. Oxygen (O2) is the final
electron acceptor at the end of the chain, and it is reduced to two water molecules by the transfer of four electrons from complex IV.
|
For bookkeeping purposes in research (see P:O ratios and respiratory control, below), the efficiency of oxidative phosphorylation is measured by dividing the amount of phosphate incorporated into ADP by the amount of atomic oxygen reduced. One atom of oxygen is reduced by two electrons (one electron pair).
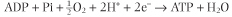
|
| IRON DEFICIENCY LEADS TO ANAEMIA |
| A 45-year-old woman complains of tiredness and appears pale. She is a vegetarian and is experiencing a monthly menstrual flow that is heavy and prolonged. Her hematocrit is 0.32 (reference range 0.36-0.46) and her hemoglobin concentration 90 g/L (normal range 120-160 g/L; 12-16 g/dL). |
| Comment. Iron deficiency anemia is a common nutritional problem and is especially common in menstruating and pregnant women because of their increased dietary requirement for iron. Men require about 1 mg iron/day, menstruating women about 2 mg/day, and pregnant women about 3 mg/day. Iron is required to maintain normal amounts of hemoglobin, the cytochromes, and iron-sulfur complexes that are central to oxygen transport and energy metabolism. All these processes are impaired in iron deficiency. Heme iron, which is found in meats, is absorbed much more readily than inorganic iron such as that found in egg yolks, vegetables, and nuts. For hematology reference values, see Table 4.2 on p. 48. |
| For each pair of electrons transported through complexes I, III, or IV, a sufficient number of protons is pumped by each complex for the synthesis of approximately one mole of ATP.
If electron transport begins with an electron pair from NADH, approximately three moles of ATP are synthesized, whereas an electron pair from any of the other three FADH2-containing flavoproteins, yields about two moles of ATP, because the proton-pumping capability of complex I is bypassed.
|
|