| Flavoproteins contain FAD or FMN prosthetic groups
|
Complex I, also called NADH-Q reductase or NADH dehydrogenase, is a flavoprotein containing FMN. It oxidizes mitochondrial NADH, and transfers electrons through FMN and iron-sulfur (FeS) complexes to ubiquinone, providing enough energy to pump four protons from the matrix in the reaction:
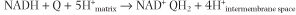
|
Three other flavoproteins transfer electrons from oxidizable substrates via FADH2 to ubiquinone (Q) (Fig. 8.5):
- succinate - Q reductase (complex II or succinate dehydrogenase of the TCA cycle) (see Chapter 13) oxidizes succinate to fumarate and reduces FAD to FADH2
- glycerol-3-phosphate - Q reductase, a part of the glycerol-3-P shuttle (see below), oxidizes cytoplasmic glycerol-3-P to dihydroxyacetone phosphate (DHAP) and reduces FAD to FADH2
- fatty acyl CoA dehydrogenase catalyzes the first step in the mitochondrial oxidation of fatty acids and also produces FADH2
|
| page 99 |  | | page 100 |
| Figure 8.6 Iron-sulfur complexes. Cys, cysteine. |
| A RARE COENZYME Q10 DEFICIENCY |
A 4-year-old boy presented with seizures, progressive muscle weakness, and encephalopathy. Accumulation of lactate, a product of anaerobic metabolism of glucose , in the cerebrospinal fluid (CSF) suggested a defect in mitochondrial oxidative metabolism. Muscle mitochondria were isolated for study. The activities of the individual Complexes I, II, III, and IV were normal, but the combined activities of I +III and II +III were significantly decreased. Treatment with coenzyme Q10 improved the muscle weakness, but not the encephalopathy. , in the cerebrospinal fluid (CSF) suggested a defect in mitochondrial oxidative metabolism. Muscle mitochondria were isolated for study. The activities of the individual Complexes I, II, III, and IV were normal, but the combined activities of I +III and II +III were significantly decreased. Treatment with coenzyme Q10 improved the muscle weakness, but not the encephalopathy. |
| Comment. Severe muscle weakness, encephalopathy, or both, may be caused in so-called mitochondrial myopathies by mitochondrial defects involving the electron transport system. The finding of increased lactate in the CSF suggests a defect in oxidative phosphorylation. The decreased activities of complexes I +III and II +III suggested a deficiency in coenzyme Q10, which was confirmed by direct measurements. |
| Both FMN and FAD contain the water-soluble vitamin riboflavin. A dietary deficiency of riboflavin can severely impair the function of these and other flavoproteins.
|
| Iron-sulfur complexes participate in redox reactions |
| Iron is an important constituent of heme proteins, such as hemoglobin, myoglobin, cytochromes, and catalase, but it is also associated with iron-sulfur (FeS) complexes or nonheme iron proteins that function as electron transporters in the mitochondrial electron transport system. The Fe2S2 and Fe4S4 types are shown in Figure 8.6. In each case, the iron-sulfur center is bound to a peptide through cysteine residues. The FeS complexes undergo reversible distortion and relaxation during redox reactions. The redox energy is said to be conserved in the 'conformational energy' of the protein. |
| TRANSFER OF ELECTRONS FROM NADH INTO MITOCHONDRIA |
| NADH is produced in the cytosol during carbohydrate metabolism. Since it cannot cross the inner mitochondrial membrane, it cannot donate electrons directly to the electron transport system. Two redox shuttles that transfer the electrons from NADH into mitochondria rather than physical transfer of the NADH solve this problem. A characteristic feature of these shuttles is that they are powered by cytoplasmic and mitochondrial isoforms of the same enzyme. The glycerol-3-P shuttle is the simpler of the two (Fig. 8.7). It transfers the electrons of NADH from the cytoplasm to mitochondrion by reducing FAD to FADH2. Cytoplasmic glycerol-3-P dehydrogenase catalyzes reduction of DHAP with NADH to glycerol-3-P. The cytoplasm-derived glycerol-3-phosphate is oxidized back to DHAP by another glycerol-3-phosphate dehydrogenase isoform of in the inner mitochondrial membrane, a flavoprotein in which FAD is reduced to FADH2. The electrons are then transferred to the common pathway via ubiquinone. The yield of ATP from cytoplasmic NADH by this pathway is approximately 2 moles, rather than the maximum of 3 moles available from mitochondrial NADH via the NADH-Q reductase complex (Complex I). |
| Many cells use the glycerol 3-P shuttle, but heart and liver rely on the malate-aspartate shuttle, which yields 3 moles of ATP per mole of NADH. This shuttle is more complicated, because the substrate, malate, can cross the inner mitochondrial membrane, but the membrane is impermeable to the product, oxaloacetate - there is no oxaloacetate transporter. The exchange is therefore accomplished by interconversion between α-keto-and α-amino acids, involving cytoplasmic and mitochondrial glutamate and α-ketoglutarate, and isozymes of glutamate-oxaloacetate transaminase (aspartate aminotransferase). |
| page 100 |  | | page 101 |
| Figure 8.7 Redox shuttles in the inner mitochondrial membrane. The glycerol phosphate and malate-aspartate shuttles. AST, aspartate aminotransferase. |
|