| Regulation of glycolysis in erythrocytes
|
| page 148 |  | | page 149 |
| INHIBITION OF ENOLASE BY FLUORIDE |
Measurement of blood glucose concentration is used for the diagnosis of diabetes. Frequently these measurements are made in the clinical laboratory more than 1 h after the collection of the blood sample. Because RBCs can metabolize glucose concentration is used for the diagnosis of diabetes. Frequently these measurements are made in the clinical laboratory more than 1 h after the collection of the blood sample. Because RBCs can metabolize glucose to lactate, even in a sealed, anaerobic vial, the glucose to lactate, even in a sealed, anaerobic vial, the glucose in blood will be consumed, with concomitant production of lactate, which will lead to acidification of the blood sample. These reactions proceed in RBCs at room temperature, so that both blood glucose in blood will be consumed, with concomitant production of lactate, which will lead to acidification of the blood sample. These reactions proceed in RBCs at room temperature, so that both blood glucose concentration and pH will decrease during standing. How can this be prevented? This is readily achieved by adding an inhibitor of glycolysis. Sulfhydryl reagents would work - they are inhibitors of GAPDH; however, most blood samples are collected with a small amount of a much cheaper reagent, sodium fluoride concentration and pH will decrease during standing. How can this be prevented? This is readily achieved by adding an inhibitor of glycolysis. Sulfhydryl reagents would work - they are inhibitors of GAPDH; however, most blood samples are collected with a small amount of a much cheaper reagent, sodium fluoride , in the sample-collection vial. Fluoride is a strong competitive inhibitor of enolase, blocking glycolysis and lactate production in the RBC. It is an unusual competitive inhibitor, since fluoride bears little resemblance to 2-phosphoglycerate. In this case, fluoride forms a complex with phosphate and Mg2+ in the active site of the enzyme, blocking access of substrate. , in the sample-collection vial. Fluoride is a strong competitive inhibitor of enolase, blocking glycolysis and lactate production in the RBC. It is an unusual competitive inhibitor, since fluoride bears little resemblance to 2-phosphoglycerate. In this case, fluoride forms a complex with phosphate and Mg2+ in the active site of the enzyme, blocking access of substrate. |
 |
RBCs consume glucose at a fairly steady rate. They are not physically active like muscle, and do not require energy for
transport of O2 or CO2. Glycolysis in red cells appears to be regulated simply by the energy needs of the cell, i.e. the requirement for ATP to maintain ion gradients, which is relatively constant. The balance between ATP consumption and production is controlled allosterically at three sites, the hexokinase, phosphofructokinase-1, and pyruvate kinase reactions (Fig. 11.2). Based on measurements of the Vmax of the various enzymes in RBC lysates in vitro, hexokinase is present at the lowest activity of all glycolytic enzymes. Its maximal activity is only about five times the rate of glucose at a fairly steady rate. They are not physically active like muscle, and do not require energy for
transport of O2 or CO2. Glycolysis in red cells appears to be regulated simply by the energy needs of the cell, i.e. the requirement for ATP to maintain ion gradients, which is relatively constant. The balance between ATP consumption and production is controlled allosterically at three sites, the hexokinase, phosphofructokinase-1, and pyruvate kinase reactions (Fig. 11.2). Based on measurements of the Vmax of the various enzymes in RBC lysates in vitro, hexokinase is present at the lowest activity of all glycolytic enzymes. Its maximal activity is only about five times the rate of glucose consumption by the RBC. However, it is also subject to feedback (allosteric) inhibition by its product Glc-6-P, and is rate-limiting for glucose consumption by the RBC. However, it is also subject to feedback (allosteric) inhibition by its product Glc-6-P, and is rate-limiting for glucose utilization in the RBC - in contrast, the glucose utilization in the RBC - in contrast, the glucose transporter GLUT-1 is present at much higher concentration and activity, representing over 5% of total RBC membrane protein. Hexokinase has 30% homology between its N and C terminal domains, the result of duplication and fusion of a primordial gene; binding of Glc-6-P to the N-terminal domain inhibits the activity of the enzyme and production of Glc-6-P at the active site in the C-terminal domain. transporter GLUT-1 is present at much higher concentration and activity, representing over 5% of total RBC membrane protein. Hexokinase has 30% homology between its N and C terminal domains, the result of duplication and fusion of a primordial gene; binding of Glc-6-P to the N-terminal domain inhibits the activity of the enzyme and production of Glc-6-P at the active site in the C-terminal domain.
|
| Figure 11.7 Allosteric regulation of phosphofructokinase-1 (PFK-1) by ATP. AMP is a potent activator of PFK-1 in the presence of ATP. |
PFK-1 is the primary site of regulation of glycolysis, controlling the flux of Fru-6-P to Fru-1,6-BP and, indirectly through the phosphoglucose isomerase reaction, the level of Glc-6-P and inhibition of hexokinase. Although present at 20 times higher concentration than hexokinase, PFK-1 activity is uniquely sensitive to the energy status of the cell. Amazingly, ATP is both a substrate and an allosteric inhibitor of PFK-1 - a dual function that permits fine control over the activity of the enzyme (Fig. 11.7). AMP and ADP relieve the
inhibition by ATP, so that the overall activity of PFK-1, and thus the rate of glycolysis, depends on the cell's (AMP + ADP)/ATP concentration ratio. These products are interconvertible by the adenylate kinase reaction:
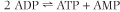
|
| When ATP is consumed and ADP increases, AMP is formed. The increasing ADP and AMP concentrations relieve the inhibition of PFK-1 by ATP, activating glycolysis. The phosphorylation of ADP during glycolysis and then of AMP by the adenylate kinase reaction gradually restores the ATP concentration or energy charge of the cell, and, as the AMP concentration declines, the rate of glycolysis decreases to a steady-state level. In general, glycolysis operates at a constant rate in the red cell, where ATP consumption is steady, but the activity of this pathway changes rapidly in response to ATP consumption (and AMP generation) in muscle during exercise.
|
| As shown in Figure 11.7, the concentration of ATP in the RBC (1-2 mmol/L), is poised at the steep point in the concentration-response curve for ATP inhibition of PFK-1. Under normal conditions, the activity of PFK-1 is heavily suppressed by ambient ATP. AMP, which is present at much lower concentration (∼0.05 mmol/L), relieves this inhibition. In effect, small fractional conversions of ATP to AMP in the RBC yield large relative increases in AMP concentration. In this way, the activity of PFK-1 in the red cell (and especially muscle) becomes exquisitely sensitive to changes in the energy status of the cell, as measured by the AMP concentration. AMP not only relieves the inhibition of PFK-1 by ATP, but also decreases the Km for the substrate Fru-6-P, further increasing the catalytic efficiency of the enzyme.
|
| page 149 |  | | page 150 |
|
Table 11-1.
Regulation of glycolysis in the red cell. |
| Body_ID: None |
| Regulation of glycolysis in the red cell |
| Body_ID: T011001.50 |
| Enzyme | Regulator |
| Body_ID: T011001.100 |
| Hexokinase | inhibited by glucose-6-P |
| Body_ID: T011001.150 |
| Phosphofructokinase-1 | inhibited by ATP; activated by AMP |
| Body_ID: T011001.200 |
| Pyruvate kinase | activated by fructose-1,6-BP |
| Body_ID: T011001.250 |
| In addition to regulation at hexokinase and PFK-1, pyruvate kinase in liver is allosterically activated by Fru-1,6-BP,
the product of the PFK-1 reaction. This process, known as feed-forward regulation, may be important in the RBC to limit the accumulation of reactive triose phosphate intermediates in the cytosol.
|
| Each of the three enzymes involved in regulation of glycolysis - hexokinase, PFK-1, and pyruvate kinase - has characteristic features of regulatory enzymes: they are dimeric or tetrameric enzymes, are present at low Vmax in comparison with other enzymes in the pathway, and catalyze irreversible reactions. The regulation of glycolysis in liver, muscle, and other tissues is more complicated than in the RBC (Table 11.1), because of greater variability in the rate of fuel consumption and the interplay between carbohydrate and lipid metabolism during aerobic metabolism. In these tissues, the amount and activity of the regulatory enzymes are regulated by other allosteric effectors, by covalent modification, and by induction or repression of enzyme activity.
|
|