| Approximately two-thirds of total body water is in the intracellular fluid (ICF), and one-third remains in the extracellular fluid (ECF). ECF consists of interstitial fluid and lymph (15% body weight), plasma (3% body weight), and transcellular fluids, which include gastrointestinal fluid, urine, CSF, and others (Fig. 22.1).
|
| The capillary wall, which separates plasma from the interstitial fluid, is freely permeable to water and electrolytes, but restricts the flow of proteins
|
| These properties of the capillary wall mean that, whereas ions and low-molecular-weight molecules are similarly distributed in the ECF and plasma, the concentration of protein is four to five times greater in plasma than in the interstitial fluid.
|
| page 315 |  | | page 316 |
| Figure 22.1 Water, sodium and potassium distribution in the body. The main water compartments in the body are the intracellular fluid (ICF) and the extracellular fluid (ECF). ECF includes interstitial fluid and plasma. A gradient of sodium and potassium concentration is maintained across cell membranes by Na+/K+-ATPase, which pumps sodium ions from ICF to the ECF, and potassium ions in the opposite direction. Sodium is a major contributor to the osmolality of the ECF, and a main determinant of the distribution of water between ECF and ICF. On the other hand, the distribution of water between plasma and interstitial fluid is determined by the oncotic pressure exerted by plasma proteins. bw, body weight. |
The total plasma concentration of cations is about 150 mmol/L, of which sodium is approximately 140 mmol/L and potassium 4 mmol/L. The most abundant plasma anions are chloride and bicarbonate, with average concentrations
100 mmol/L and 25 mmol/L, respectively (Fig. 22.2). The rest of the anions are, for the purposes of electrolyte balance, considered together as constituting the so-called anion gap which is calculated as follows:
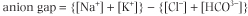
|
| 'Anion gap' is a somewhat artificial term which came into existence because anions, which include phosphate, sulfate, protein, and organic anions such as lactate, citrate, pyruvate, acetoacetate, and 3-hydroxybutyrate, are not measured by the clinical laboratories as frequently as the 'main' electrolytes. The anion gap is normally about 10 mmol/L. It may increase several-fold in conditions where inorganic and organic anions accumulate, e.g., in renal failure or diabetic ketoacidosis, and for this reason it is diagnostically important.
|
Figure 22.2 Ions in the plasma and in the intracellular fluid. The most important ions in plasma are sodium, potassium, calcium, chloride, phosphate, and bicarbonate. Sodium chloride , in a concentration close to 0.9%, is the main ionic component of the ECF. Potassium is the main intracellular cation. , in a concentration close to 0.9%, is the main ionic component of the ECF. Potassium is the main intracellular cation. |
Figure 22.2 In addition to the electrolytes shown here, glucose and urea and urea contribute to plasma osmolality. Normally their contribution is small, because they are present in plasma at relatively low molar concentrations (about 5 mmol/L each). The contribution of glucose contribute to plasma osmolality. Normally their contribution is small, because they are present in plasma at relatively low molar concentrations (about 5 mmol/L each). The contribution of glucose to osmolality becomes important in diabetes, when its concentration increases: because glucose to osmolality becomes important in diabetes, when its concentration increases: because glucose is confined to the ECF, glucose is confined to the ECF, glucose excess induces movement of water from the cells to the ECF. The plasma concentration of urea excess induces movement of water from the cells to the ECF. The plasma concentration of urea increases in renal failure; however, because it can cross cell membranes freely, it does not contribute to water movement between ECF and ICF. increases in renal failure; however, because it can cross cell membranes freely, it does not contribute to water movement between ECF and ICF. |
| Figure 22.2 The main intracellular cation is potassium. There is also a substantial amount of magnesium in cells. The main anions in the ICF are phosphates and proteins. |
| In the intracellular fluid (ICF), the main cation is potassium. Its concentration in this fluid is about 110 mmol/L, which is almost 30-fold greater than that in the ECF. The concentrations of sodium and chloride in the ICF are only
10 mmol/L and 4 mmol/L, respectively. Anions present in the ICF include proteins, phosphate, and other substances that cannot diffuse freely through the cell membranes.
|
| Water diffuses freely across most cell membranes, but the movement of ions and neutral molecules is restricted
|
| page 316 |  | | page 317 |
| Figure 22.3 Sodium-potassium pump. The enzyme, Na+/K+-ATPase, is responsible for maintaining sodium and potassium concentration gradients across cell membranes. It also has a key role in the reabsorption of sodium in the kidney tubules. |
The transport of small molecules across otherwise impermeable membranes is made possible because of the existence
of specific transport proteins, including ion pumps. Of the several ion pumps, the sodium/potassium ATPase (Na+/K+ATPase), also referred to as the sodium-potassium pump, shows the greatest transport activity. Its hydrolyzes one ATP molecule, and the released energy drives the transfer of three sodium ions from the cell to the outside, and, in turn, two potassium ions from the outside into the cell. Thus this pump maintains chemical and electrical potential gradients between the inside and the outside of the cell (Fig. 22.3). For most cells, the membrane potential ranges from 50 to 90 mV, being negative inside the cell. The electrochemical gradient is a source of energy for the transport of many substances, in particular for the cotransport of sodium ions with glucose , amino acids , amino acids , and phosphate (see Chapter 7; the role of ion gradients in nerve transmission is described in Chapter 39). , and phosphate (see Chapter 7; the role of ion gradients in nerve transmission is described in Chapter 39).
|
|