| THE RENIN-ANGIOTENSIN SYSTEM
|
| The renin-angiotensin system is important in the control of blood pressure and the vascular tone
|
| Figure 22.14 The renin-angiotensin system. VSMC, vascular smooth muscle cells; CNS, central nervous system. *AT-1 receptor blocked by e.g. losartan. **AT-2 receptor blocked by saralasin. |
| page 326 |  | | page 327 |
| RENIN-ANGIOTENSIN SYSTEM AND CARDIAC FAILURE |
A 65-year-old man with a previous anterior myocardial infarction presented with increasing fatigue, shortness of breath and ankle edema. Physical examination showed mild tachycardia, a raised jugular venous pressure and some ankle oedema. An echocardiogram showed that the function of the left ventricle during systole was very poor. The patient's serum measurements revealed: sodium 140 mmol/L, potassium 3.5 mmol/L, protein 34 (normal 35-45), g/dL, creatinine 80 μmol/L (0.90 mg/dL), and urea 7.5 mmol/L (45 mg/dL). 7.5 mmol/L (45 mg/dL). |
| Comment. This man presents with symptoms and signs of cardiac failure. The impaired function of the heart leads to a decreased blood flow through the kidney, activation of the renin-angiotensin system and stimulation of aldosterone secretion. Aldosterone causes an increased renal reabsorption of sodium and water retention, thereby increasing extracellular fluid volume and causing edema. |
Renin is an enzyme produced principally in the juxtaglomerular apparatus of the kidney; it is stored in secretory granules and released in response to decreased renal perfusion pressure. Renin is a protease that uses angiotensinogen as its substrate. Angiotensinogen is a glycoprotein of more than 400 amino acids , is synthesized in the liver, and has a variable structure and molecular weight. Renin cleaves the decapeptide angiotensin I from angiotensinogen. Angiotensin I is a short, 10-amino-acid peptide. It is a substrate for peptidyl-dipeptidase A (angiotensin converting enzyme; ACE). ACE removes two amino acids , is synthesized in the liver, and has a variable structure and molecular weight. Renin cleaves the decapeptide angiotensin I from angiotensinogen. Angiotensin I is a short, 10-amino-acid peptide. It is a substrate for peptidyl-dipeptidase A (angiotensin converting enzyme; ACE). ACE removes two amino acids from angiotensin I, producing the most potent known vasoconstrictor
molecule, angiotensin II. ACE is particularly abundant in the pulmonary blood vessels where it is bound to the luminal surface of the endothelial cells. Drugs that inhibit ACE are now extensively used in the treatment of hypertension and in cardiology (Fig. 22.15). from angiotensin I, producing the most potent known vasoconstrictor
molecule, angiotensin II. ACE is particularly abundant in the pulmonary blood vessels where it is bound to the luminal surface of the endothelial cells. Drugs that inhibit ACE are now extensively used in the treatment of hypertension and in cardiology (Fig. 22.15).
|
| Angiotensin II constricts vascular smooth muscle, thereby increasing blood pressure and reducing renal blood flow and glomerular filtration rate. It also stimulates aldosterone secretion. These actions of angiotensin II are mediated through membrane receptor (AT1) which signals through G-proteins and phospholipase C. This action is antagonized through the AT2 receptor class which promotes vasodilatation and renal sodium loss.
|
| Apart from the classical pathway of production of angiotensin II from angiotensin I by the action of ACE, there is an alternative pathway leading directly from angiotensinogen to angiotensin II. Substantial amounts of angiotensin II are formed in the kidney. Juxtaglomerular cells contain ACE, angiotensin I and angiotensin II. Angiotensin II is also synthesized in the glomerular and tubular cells and is secreted into the tubular fluid and interstitial space. Angiotensin II receptors are present on the tubular and renal vascular cells: therefore locally produced angiotensin II probably influences tubular reabsorption and renal vascular tone through autocrine and paracrine action.
|
| Aldosterone regulates sodium reabsorption
|
|
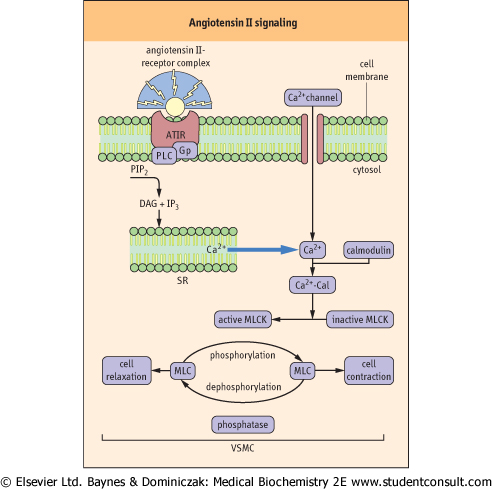
| Figure 22.15 Cellular mechanism of Angiotensin II-induced vasoconstriction. The angiotensin receptor (AT1) is linked to G-proteins. The binding of angiotensin II to the receptor leads to phospholipase C-mediated formation of inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG). This results in the mobilization of Ca2+ from the sarcoplasmic reticulum (SR), and to the cell entry of the extracellular Ca2+ which takes place as a result of the activation of calcium channels. The increase in the cytosolic Ca2+ initiates the contractile response of the vascular smooth muscle cells (VSMC) and Ca2+ subsequently binds to calmodulin (Cal). The Ca2+-calmodulin complex activates myosin light-chain kinase (MLCK) which phosphorylates myosin light chains and elicits muscular tension. This effect is terminated by the dephosphorylation of myosin (see also Chapter 19). AT1 receptor antagonists, such as losartan, inhibit the vasoconstrictor effects of angiotensin II and are used in the treatment of hypertension. |
| page 327 |  | | page 328 |
ACUTE RENAL FAILURE LEADS TO A STEEP FALL IN OUTPUT OF URINE, AND CAUSES HIGH SERUM UREA AND CREATININE CONCENTRATIONS AND CREATININE CONCENTRATIONS |
A 25-year-old man was admitted to hospital unconscious after a motorcycle accident. He had evidence of shock with hypotension and tachycardia, a fractured skull and multiple injuries to his limbs. Despite treatment with intravenous colloid and blood he showed persistent oliguria (5-10ml/hour: oliguria is <20ml/hour). Oliguria due to acute tubular necrosis may be distinguished from prerenal azotemia by measuring urine osmolality (>500mOsm/kg in prerenal azotemia and <350 mOsm/kg in acute renal failure) also urine sodium concentration (<20mmol/L in prerenal azotemia and >40 mmol/L in acute renal failure). On the third day, his serum creatinine concentration had risen to 300 μmol/L (3.9 mg/dL) and his urea concentration is to mmol/L (132 mg/dL). Reference values are: concentration is to mmol/L (132 mg/dL). Reference values are:
- creatinine: 20-80 μmol/L (0.23-0.90 mg/dL)
- urea: 2.5-6.5 mmol/L (16.2-39 mg/dL)
|
Comment. This young man has developed acute renal failure due to acute tubular necrosis as a consequence of hypovolaemic shock. He subsequently underwent emergency haemofiltration. Renal function started to recover after 2 weeks with an initial increased urine volume, the so-called 'diuretic phase').
- urea
 mg/dL = mmol/L × 6.02 mg/dL = mmol/L × 6.02 - creatinine mg/dL = μmol/L × 0.0113
|
| Aldosterone is produced in the adrenal cortex, and is a major mineralocorticosteroid hormone in man. Aldosterone regulates the extracellular volume and controls potassium
homeostasis. These effects are mediated by binding to the cytosolic mineralocorticoid receptor in the epithelial cells, principally in the renal collecting duct. The receptor moves to the nucleus and binds to specific domains on targeted genes, altering gene expression. This leads to modification of the activities of the sodium channel and the Na+/K+-ATPase, resulting in increased sodium transport across the cell membrane. Aldosterone increases sodium reassertion,
and potassium and hydrogen ion excretion in the distal tubule.
|
|