| THE NATURE OF OXYGEN RADICAL DAMAGE
|
| The hydroxyl radical is, by far, the most damaging ROS
|
| The hydoxyl radical reacts with biomolecules primarily by hydrogen abstraction and addition reactions. Characteristic products, described as biomarkers of oxidative stress, are formed in these reactions. One of the most sensitive sites of free radical damage is the cell membrane, which is rich in readily oxidized polyunsaturated fatty acids (PUFA). Peroxidative damage to cell membranes affects the integrity and function of the membrane, compromising the cell's ability to maintain ion gradients and membrane phospholipid asymmetry.
|
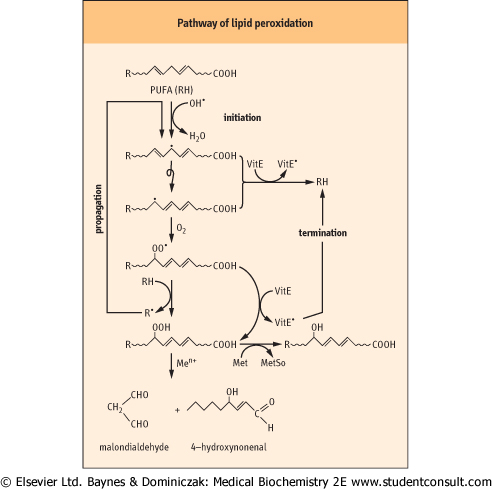
| As shown in Fig. 35.5, OH• abstracts a hydrogen atom from a PUFA, setting off a chain of lipid peroxidation reactions. Lipid peroxides formed in this reaction degrade to form characteristic products, such as malondialdehyde (MDA) and hydroxynonenal (HNE). These compounds react with proteins to form adducts and crosslinks, known as advanced lipoxidation end-products (ALE). MDA and HNE adducts to lysine residues have been measured in lipoproteins in the vascular wall in atherosclerosis (Chapter 17) and in neuronal plaque in Alzheimer's disease (Chapter 42), implicating oxidative stress and damage in the pathogenesis of these diseases.
|
Hydroxyl radicals also react by addition to phenylalanine, tyrosine and nucleic acids to form characteristic hydroxylated derivatives and crosslinks (Fig. 35.6). Other ROS and RNS leave tell-tale tracks, such as nitro- and chloro-tyrosine, formed from ONOOH and HOCl, respectively, and methionine sulfoxide, formed by reaction of H2O2 or HOCl with methionine sulfoxide, formed by reaction of H2O2 or HOCl with methionine residues in proteins (Fig. 35.6). Nitrotyrosine, like ALEs, is increased in atherosclerotic and Alzheimer's plaques. residues in proteins (Fig. 35.6). Nitrotyrosine, like ALEs, is increased in atherosclerotic and Alzheimer's plaques.
|
| page 500 |  | | page 501 |
|
Figure 35.5 Pathway of lipid peroxidation. OH• attacks PUFA, forming a carbon-centered lipid radical. The radical rearranges to form a conjugated dienyl radical. This radical reacts with ambient O2, forming a hydroperoxyl radical, which then abstracts a hydrogen from a neighboring lipid, forming a lipid peroxide and starting a chain reaction. This reaction continues until the supply of PUFA is exhausted, unless a termination reaction occurs. Vitamin E (discussed below) is the major chain-terminating antioxidant in membranes; it reduces both the conjugated dienyl and hydroperoxyl radicals, quenching the chain or cycle of lipid peroxidation reactions. Lipid peroxides may also be reduced by methionine (discussed below) is the major chain-terminating antioxidant in membranes; it reduces both the conjugated dienyl and hydroperoxyl radicals, quenching the chain or cycle of lipid peroxidation reactions. Lipid peroxides may also be reduced by methionine residues in lipoproteins, forming methionine residues in lipoproteins, forming methionine sulfoxide and lipid alcohols. Otherwise, they decompose to form a range of 'reactive carbonyl species' such as malondialdehyde and hydroxynonenal, which react with protein to form advanced lipoxidation end-products (ALE), which are biomarkers of oxidative stress. The reaction scheme shown here for PUFA also occurs with intact phospholipids and cholesterol esters in lipoproteins and cell membranes. sulfoxide and lipid alcohols. Otherwise, they decompose to form a range of 'reactive carbonyl species' such as malondialdehyde and hydroxynonenal, which react with protein to form advanced lipoxidation end-products (ALE), which are biomarkers of oxidative stress. The reaction scheme shown here for PUFA also occurs with intact phospholipids and cholesterol esters in lipoproteins and cell membranes. |
| ROS also react with carbohydrates to form dicarbonyl compounds that react with protein to form crosslinks and
adducts, known as glycoxidation products or advanced glycation end-products (AGE). AGE are increased in tissue proteins in diabetes as a result of hyperglycemia and oxidative stress, and the increase in chemical modification of proteins by AGE and ALE is implicated in the development of diabetic vascular, renal, and retinal complications (see also Chapter 42).
|
| SENTINEL FUNCTION OF METHIONINE |
Methionine (Met) residues are located on the surface of proteins and are easily oxidized to methionine sulfoxide (MetSO) by H2O2 or HOCl. Met is generally on the surface of proteins and rarely has a role in the active site mechanism of action of enzymes. However, there is evidence that Met serves as an 'antioxidant pawn', protecting the active site of enzymes. Half of the Met residues of glutamine synthetase could be oxidized without affecting the enzyme's specific activity. These residues were physically arranged in an array that guarded the entrance to the active site. MetSO can be reduced back to methionine sulfoxide (MetSO) by H2O2 or HOCl. Met is generally on the surface of proteins and rarely has a role in the active site mechanism of action of enzymes. However, there is evidence that Met serves as an 'antioxidant pawn', protecting the active site of enzymes. Half of the Met residues of glutamine synthetase could be oxidized without affecting the enzyme's specific activity. These residues were physically arranged in an array that guarded the entrance to the active site. MetSO can be reduced back to methionine by the enzyme methionine by the enzyme methionine sulfoxide reductase, providing a catalytic amplification of the antioxidant potential of each methionine sulfoxide reductase, providing a catalytic amplification of the antioxidant potential of each methionine residue. In addition to its scavenging role, there is evidence that oxidation of Met may be involved in the regulation of enzyme activity and targeting enzymes for proteolytic degradation. residue. In addition to its scavenging role, there is evidence that oxidation of Met may be involved in the regulation of enzyme activity and targeting enzymes for proteolytic degradation. |
| GLUTATHIONYLATION OF PROTEIN |
| S-glutathionylation or S-glutathiolation of proteins describes the formation of a disulfide bond between GSH and an -SH group on a target protein. S-thiolation of proteins is induced by both ROS and RNS and is thought to have a dual role in protecting cysteine against irreversible oxidation during oxidative stress and in modulating cellular metabolism (redox regulation). Target proteins include a wide range of regulatory enzymes with active site -SH groups, such as protein kinases and transcription factors. S-thiolation also appears to protect proteins against ubiquitin-mediated proteasomal degradation. It is reversed by nonenzymatic reduction by GSH or by enzymes using thiol protein cofactors (thioredoxin, glutaredoxin). |
|