| AGING OF MUSCLE - DAMAGE TO MITOCHONDRIAL DNA
|
Old age is characterized by a general decrease in skeletal muscle mass (sarcopenia) and strength, as a result of a decrease in both the number of motoneurons and the number and size of myofibers. The fiber loss is accompanied by an increase in interstitial, fibrous connective tissue, and a reduction in capillary density, which limits the blood supply. The decrease in muscle mass and strength contributes to frailty and the increased risk of mortality. The loss in skeletal muscle mass may also contribute to glucose intolerance in the elderly as a result of the decreasing mass of tissue available to take up glucose intolerance in the elderly as a result of the decreasing mass of tissue available to take up glucose from blood. from blood.
|
One of the major changes in muscle biochemistry with age in an increase in the number of muscle cells with mitochondria deficient in cytochrome oxidase, which limits the muscle's ability to do work. As mitochondria become less efficient in oxidizing NADH, they become more reduced, and the accumulation of partially reduced ubiquinone (semiquinone) promotes the reduction of molecular oxygen, leading to increased superoxide production in older mitochondria. Under these conditions, when oxidative phosphorylation is impaired, cells appear to generate ATP primarily by glycolysis. NADH is oxidized extramitochondrially, primarily by NADH oxidases in the plasma membrane, which produce hydrogen peroxide, but no ATP. NADH oxidase:
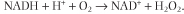
|
| These changes are observed in both cardiac and skeletal muscle and appear to result from major, random deletions in mitochondrial DNA (25-75% of total mtDNA), which are then amplified by clonal expansion, leading to fiber atrophy and breakage. The muscle fiber is only as strong as its weakest link, so that small regions of fiber loss affect overall muscle capacity. Fortunately, sarcopenia can be delayed and partially reversed by resistance exercise, thus the emphasis on regular exercise among the elderly.
|
|