Classification of amino acids based on chemical structure based on chemical structure
|
The properties of each amino acid are dependent on its side chain (-R); the side chains are the functional groups that are the major determinants of the structure and function of proteins, as well as the electrical charge of the molecule. Knowledge of the properties of these side chains is important for understanding methods of analysis, purification, and identification of proteins. Amino acids with charged, polar, or hydrophilic side chains are usually exposed on the surface of proteins. The nonpolar hydrophobic residues are usually buried in the hydrophobic interior or core of a protein and are out of contact with water. The 20 amino acids with charged, polar, or hydrophilic side chains are usually exposed on the surface of proteins. The nonpolar hydrophobic residues are usually buried in the hydrophobic interior or core of a protein and are out of contact with water. The 20 amino acids in proteins encoded by DNA are listed in Table 2.1 and are classified according to their side-chain functional groups. in proteins encoded by DNA are listed in Table 2.1 and are classified according to their side-chain functional groups.
|
| page 7 |  | | page 8 |
Figure 2.1 Structure of an amino acid. Except for glycine , four different groups are attached to the α-carbon of an amino acid. Table 2.1 on this page lists the structures of the R moiety. , four different groups are attached to the α-carbon of an amino acid. Table 2.1 on this page lists the structures of the R moiety. |
Figure 2.2 Enantiomers. The mirror-image pair of amino acids . Each amino acid represents nonsuperimposable mirror images. The mirror-image stereoisomers are called enantiomers. . Each amino acid represents nonsuperimposable mirror images. The mirror-image stereoisomers are called enantiomers. |
Some amino acids occur in free or combined states, but not in proteins. Measurement of abnormal amino acids occur in free or combined states, but not in proteins. Measurement of abnormal amino acids in urine (aminoaciduria) is useful for clinical diagnosis (see Chapter 18). In plasma, free amino acids in urine (aminoaciduria) is useful for clinical diagnosis (see Chapter 18). In plasma, free amino acids are usually found in the order of 10 to 100 μmol/L, including many that are not found in protein. Citrulline, for example, is an important metabolite of L-arginine and a product of nitric oxide are usually found in the order of 10 to 100 μmol/L, including many that are not found in protein. Citrulline, for example, is an important metabolite of L-arginine and a product of nitric oxide synthase, an enzyme that produces nitric oxide synthase, an enzyme that produces nitric oxide , an important vasoactive signaling molecule. Urinary amino acid concentration is usually expressed as μmol/g creatinine. Creatinine is an amino acid derived from muscle, and is excreted in relatively constant amounts per unit body mass per day. Thus, the creatinine concentration in urine, normally about 1 mg/mL, can be used to correct for urine dilution. The most abundant amino acid in urine is glycine , an important vasoactive signaling molecule. Urinary amino acid concentration is usually expressed as μmol/g creatinine. Creatinine is an amino acid derived from muscle, and is excreted in relatively constant amounts per unit body mass per day. Thus, the creatinine concentration in urine, normally about 1 mg/mL, can be used to correct for urine dilution. The most abundant amino acid in urine is glycine , which is present as 400-2000 μg/g creatinine. , which is present as 400-2000 μg/g creatinine. |
|
Table 2-1.
The 20 amino acids found in proteins. |
| Body_ID: None |
| The 20α-amino acids specified by the genetic code |
| Body_ID: T002001.50 |
Amino acids | Structure of R moiety |
| Body_ID: T002001.100 |
Aliphatic amino acids |
| Body_ID: T002001.150 |
glycine (Gly, G) (Gly, G) | -H |
| Body_ID: T002001.200 |
| alanine (Ala, A) | -CH3 |
| Body_ID: T002001.250 |
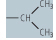
| valine (Val, V) |
 |
| Body_ID: T002001.300 |
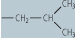
| leucine (Leu, L) |
 |
| Body_ID: T002001.350 |
| isoleucine (Ile, I) |
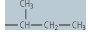 |
| Body_ID: T002001.400 |
Sulfur-containing amino acids |
| Body_ID: T002001.450 |
| cysteine (Cys, C) | -CH2-SH |
| Body_ID: T002001.500 |
methionine (Met, M) (Met, M) | -CH2-CH2-S-CH3 |
| Body_ID: T002001.550 |
Aromatic amino acids |
| Body_ID: T002001.600 |
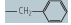
| phenylalanine (Phe, F) |
 |
| Body_ID: T002001.650 |
| tyrosine (Tyr, Y) |
 |
| Body_ID: T002001.700 |
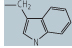
| tryptophan (Trp, W) |
 |
| Body_ID: T002001.750 |
| Imino acid |
| Body_ID: T002001.800 |
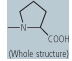
| proline (Pro, P) |
 |
| Body_ID: T002001.850 |
Neutral amino acids |
| Body_ID: T002001.900 |
| serine (Ser, S) | -CH2-OH |
| Body_ID: T002001.950 |
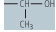
| threonine (Thr, T) |
 |
| Body_ID: T002001.1000 |
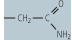
| asparagine (Asn, N) |
 |
| Body_ID: T002001.1050 |
| glutamine (Gln, Q) |
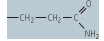 |
| Body_ID: T002001.1100 |
Acidic amino acids |
| Body_ID: T002001.1150 |
| aspartic acid (Asp, D) | -CH2-COOH |
| Body_ID: T002001.1200 |
| glutamic acid (Glu, E) | -CH2-CH2-COOH |
| Body_ID: T002001.1250 |
Basic amino acids |
| Body_ID: T002001.1300 |
| histidine (His, H) |
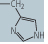 |
| Body_ID: T002001.1350 |
| lysine (Lys, K) | -CH2-CH2-CH2-CH2-NH2 |
| Body_ID: T002001.1400 |
| arginine (Arg, R) |
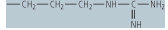 |
| Body_ID: T002001.1450 |
|
| Body_ID: T002001.1500 |
The three-letter and single-letter abbreviations in common use are given in parentheses.
|
|