| Ionization state of an amino acid
|
Amino acids are amphoteric molecules - they have both basic and acidic groups. Monoamino and monocarboxylic acids are ionized in different ways in solution, depending on the solution pH. At pH 7, the 'zwitterion' +H3N-CH2-COO- is the dominant species of glycine in solution, and the overall molecule is therefore electrically neutral. On titration to acidic pH, the α-amino group is protonated and positively charged, yielding the cation +H3N-CH2-COOH, while titration with alkali yields the anionic H2N-CH2-COO- species. in solution, and the overall molecule is therefore electrically neutral. On titration to acidic pH, the α-amino group is protonated and positively charged, yielding the cation +H3N-CH2-COOH, while titration with alkali yields the anionic H2N-CH2-COO- species.
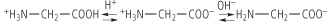
|
pKa values for the α-amino and α-carboxyl groups and side chains of acidic and basic amino acids are shown in Table 2.3. The overall charge on a protein depends on the contribution from basic (positive charge) and acidic (negative charge) amino acids are shown in Table 2.3. The overall charge on a protein depends on the contribution from basic (positive charge) and acidic (negative charge) amino acids , but the actual charge on the protein varies with the pH of the solution. To understand how the side chains affect the charge on proteins, it is worth recalling the Henderson-Hasselbalch equation. , but the actual charge on the protein varies with the pH of the solution. To understand how the side chains affect the charge on proteins, it is worth recalling the Henderson-Hasselbalch equation.
|
| page 10 |  | | page 11 |
|
Table 2-3.
pK values and ionized groups in proteins. |
| Body_ID: None |
| pK values and ionized groups in proteins |
| Body_ID: T002003.50 |
| Group | Acid (protonated form) (conjugate acid) | H++Base (unprotonated form) (conjugate base) | pKa |
| Body_ID: T002003.100 |
| terminal carboxyl residue (α-carboxyl) | -COOH (carboxylic acid) | -COO-+H+(carboxylate) | 3.0-5.5 |
| Body_ID: T002003.150 |
| aspartic acid (β-carboxyl) | -COOH | -COO-+H+ | 3.9 |
| Body_ID: T002003.200 |
| glutamic acid (γ-carboxyl) | -COOH | -COO-+H+ | 4.3 |
| Body_ID: T002003.250 |
| histidine (imidazole) | | | 6.0 |
| Body_ID: T002003.300 |
| terminal amino (α-amino) | -NH+3 (amino) | -NH2 +H+(amine) | 8.0 |
| Body_ID: T002003.350 |
| cysteine (sulfhydryl) | -SH (thiol) | -S-+H+(thiolate) | 8.3 |
| Body_ID: T002003.400 |
| tyrosine (phenolic hydroxyl) | | | 10.1 |
| Body_ID: T002003.450 |
| lysine (ε-amino) | -NH+3 (ε-amino) | -NH2 +H+(ε-amine) | 10.5 |
| Body_ID: T002003.500 |
| arginine (guanidino) | | | 12.5 |
| Body_ID: T002003.550 |
|
| Body_ID: T002003.600 |
pKa indicates the approximate value, because it depends on temperature, buffer, etc.
|
|