Buffers are solutions that minimize a change in [H+], i.e. pH, on addition of acid or base. A buffer solution, containing a weak acid or weak base and a counter-ion, has maximal buffering capacity at its pKa when the acidic and basic forms are present at equal concentrations. The acidic, protonated form reacts with added base, and the basic unprotonated form neutralizes added acid, as shown below for an amino compound:
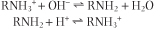
|
| An alanine solution (Fig. 2.4) has maximal buffering capacity at pH 2.4 and 9.8, i.e. at the pKa of the -COO- and NH2 groups, respectively. When dissolved in water, alanine exists as a dipolar ion, or zwitterion, in which the carboxyl group is unprotonated (-COO-) and the amino group is protonated (-NH3+). The pH of the solution is 6.1, the pI, half-way between the pKa of the amino and carboxyl groups. The titration curve of alanine by NaOH (Fig. 2.4) illustrates that alanine has minimal buffering capacity at its pI, and maximal buffering capacity at a pH equal to the pKa1 or pKa2.
|
|