
Home
Exam Guide
FAQ
Features
Glossary
Companion Notes
Just Ask Antoine!
Resources
Slide Index
Toolbox
Tutorial Index
The molecular basis for flavor
If you've ever fumbled with a ring of nearly identical keys, you understand that a subtle difference in an object's shape can make a large difference in the way it functions. Living things recognize molecules in much the same way that locks "recognize" keys- by shape. We've seen that altering a drug molecule's shape changes the way it fits into receptors. If the drug molecule binds too tightly, or if it isn't shaped in a way that allows cleanup enzymes to pull it from the receptor, it may be much more potent than the natural molecular key it imitates.
A similar lock-and-key type of model has been used to explain why different substances have different flavors. The stereochemical theory of odor suggests that a molecule that fits into an olfactory receptor can fire nerve cells, ultimately producing a particular odor perception.
Five basic odors were associated with different molecular shapes. Football shaped molecules fit in to a "camphoraceous" receptor, and smell like mothballs. Necklace-shaped molecules have a musky odor because they fit into a "musky" receptor. Wedged-shaped molecules have a pepperminty odor, tadpole-shaped molecules smell like flowers, and long thin ether molecules are, well, ethereal.
Putrid and pungent smells were explained on the basis of partial charges on atoms within the molecule, rather than by shape alone. Putrid molecules have a buildup of negative charge somewhere in the molecule that's strongly attracted to a partially positive site on the "putrid" receptor. Pungent molecules (like acetic acid, in vinegar) are just the opposite: they have an electron-deficient region that is strongly attracted to an electron-rich site on the "pungent" receptor.
These seven receptors were believed to be the only letters in the olfactory alphabet in Amoore's version of the theory, published in the early 1970's. Molecules that can lock into more than one receptor have more complex odors. For example, Amoore explained the almondy odor of benzaldehyde by showing that it could fit comfortably into the postulated shapes for the camphoraceous, floral, and pepperminty receptors.
Amoore's stereochemical theory is now known to be an oversimplification, but it's still useful in relating smells to molecular shapes. There are over a thousand olfactory receptors, not just seven. The molecule's ability to move through tissue containing layer after layer of receptors also determines how its odor is perceived. For example, attaching a hydrocarbon tail to a molecule improves its solubility in fats and alters its behavior at cell membranes. Perfume chemists have long known that adding a hydrocarbon tail to some perfume molecules increases their potency.
Let's look at some specific examples. The vanilloids (vanillin, eugenol, zingerone, and capsaicin) are molecules with distinctive flavors but obviously similar molecular structures. All contain a six-sided hexagonal ring of carbons (called a benzene ring). Subtle changes in the sizes or positions of groups of atoms attached to the ring dramatically change the compound's flavor.
Vanillin
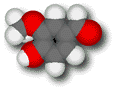 Vanillin has a soothing,
pleasant aroma. Its molecular weight is relatively low, and it is fairly volatile. Cooking
with vanilla vaporizes some of the vanillin molecules and fills the room with its aroma.
Vanillin has a soothing,
pleasant aroma. Its molecular weight is relatively low, and it is fairly volatile. Cooking
with vanilla vaporizes some of the vanillin molecules and fills the room with its aroma.
Molecules containing only carbon and hydrogen are mostly insoluble in water. The oxygen-containing groups attached to the ring in vanillin can form strong hydrogen bonds with water, making it water soluble (about a gram of vanillin can be dissolved in 100 mL of cold water). Vanillin's solubility in water is responsible for the "finish" acquired by wines aged in oak casks. Vanilla present in the wood lignin of the wine barrels slowly leaches into the wine over time.
Eugenol
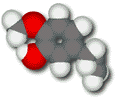 Eugenol is found in bay leaves, allspice, and oil of cloves.
Eugenol is found in bay leaves, allspice, and oil of cloves.
Eugenol has a short hydrocarbon chain attached to the ring, which makes it much less water-soluble than vanillin. Although it is practically insoluble in water, it freely mixes with fats and oils. Its fat solubility allows it to penetrate tissues and bind more tightly to the vanilloid receptor, which is believed to have a fatty side chain. The tail gives eugenol a stronger odor than vanillin has. One bay leaf is enough to season a pot of soup; more than one or two ground cloves overpower a pumpkin pie.
Eugenol has a numbing, analgesic effect. It is used as a dental antiseptic (it's one component of that strange smell some dentist's offices have). Why is the molecule an antiseptic? Apparently the hydrocarbon tail in combination with the polar OH group on the ring make eugenol rather soap-like, and it can disrupt the cell membranes of bacteria the way soap disrupts a spot of grease.
Zingerone
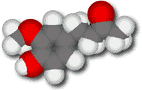 Zingerone puts the zing in
ginger and is also a flavor ingredient in mustard oil.
Zingerone puts the zing in
ginger and is also a flavor ingredient in mustard oil.
The hydrocarbon tail attached to its vanillin foundation ring doesn't lower the solubility of zingerone much because it contains a carbonyl group (C=O) that can form strong hydrogen bonds with water molecules. Zingerone is sparingly soluble in water, but also freely soluble in fats and oils.
The higher molecular weight of zingerone in combination with the polar side-chain carbonyl group makes zingerone molecules attract each other more strongly than eugenol and vanillin molecules do. As a result, zingerone is less volatile than either eugenol or vanillin. The odor of ginger isn't strong, but the hydrocarbon tail gives it a more intense flavor when it does come into contact with its receptor.
Ginger root is a popular folk medicine. Some of the beneficial medicinal qualities claimed for ginger may stem from zingerone's effectiveness as an antioxidant. Zingerone reacts with free radicals that can cause tissue damage and inflammation. Studies by researchers at Case Western University show that a topically applied extract containing zingerone may help prevent some skin cancers.
Capsaicin
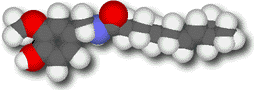 Most (though not
all) of the hot in hot peppers comes from capsaicin and a closely related compound,
dihydrocapsaicin. It occurs in much lower quantities in oregano, cinnamon, and cilantro.
Most (though not
all) of the hot in hot peppers comes from capsaicin and a closely related compound,
dihydrocapsaicin. It occurs in much lower quantities in oregano, cinnamon, and cilantro.
The compound's molecular weight is the highest of any of the vanilloids we've looked at so far, and the side chain contains a polar amide (-NHCO-) group. That makes capsaicin's volatility very low, and it is completely odorless. (A very good thing!)
Even without a telltale fragrance, capsaicin's presence in foods is hard to miss. A solution that contains only 10 parts per million produces a persistent burning sensation when placed on the tongue. It is tasteable at much lower concentrations. The intense flavor results from the molecule's long hydrocarbon tail. The chain allows it to bind very strongly with its lipoprotein receptor, which has some hydrocarbon side chains of its own (like dissolves like!) The fatty tail also allows the molecule to slip through lipid-rich cell membranes, making the burn more pervasive and persistent.
Several capsaicin-like compounds found in chiles have slight structural variations in the hydrocarbon tail, which changes their ability to bind to the receptors and their ability to penetrate layers of receptors on the tongue, mouth, and throat. That may explain why some chiles burn in the mouth, while others burn deep in the throat.
The perception that peppers are "hot" is not an accident. The capsaicin key opens a door in the cell membrane that allows calcium ions to flood into the cell. That ultimately triggers a pain signal that is transmitted to the next cell. When the cells are exposed to heat, the same events occur. Chile burns and heat burns are similar at the molecular, cellular, and sensory levels.
One expects that the long hydrocarbon tail will make capsaicin less water soluble than vanillin. This is indeed the case. Capsaicin is insoluble in cold water, but freely soluble in alcohol and vegetable oils. This is why drinking water after munching an habanero pepper won't stop the burning. A cold beer is the traditional remedy, but the small percentage of alcohol will not wash away much capsaicin. For relief from a chile burn, drink milk. Milk contains casein, a lipophilic (fat-loving) substance that surrounds and washes away the fatty capsaicin molecules in much the same way that soap washes away grease.
High concentrations are toxic. Exposure is painful and even incapacitating. Capsaicin prevents nerve cells from communicating with each other by blocking the production of certain neurotransmitters; at high concentrations it destroys the cells! Capsaicin's toxicity makes chiles more than just a food- they're also a weapon. The Mayans burned chiles to create a stinging smoke screen, and threw gourds filled with pepper extract in battle. Nowadays, capsaicin is the active ingredient in pepper sprays, used to ward off attacking muggers, dogs, and bears.
Paradoxically, capsaicin's ability to cause pain makes it useful in alleviating pain. Exposure to capsaicin lowers sensitivity to pain, and it is applied as a counter irritant in the treatment of arthritis and other chronically painful conditions.
People that eat lots of spicy capsaicin-rich foods build up a tolerance to it. The incentive: a small jolt of capsaicin excites the nervous system into producing endorphins, which promote a pleasant sense of well-being. The endorphin lift makes spicy foods mildly addictive (and for some, an obsession).
References
The Theory of Odor
- Stereochemical and vibrational theories of odour
- John Amoore's seminal work is summarized in Nature, vol 233:270-271, 1971.
- FlavorNet (Cornell U.)
- What makes popcorn smell like popcorn? Get the answers at Cornell University's FlavorNet, a molecular structure database indexed by flavor. To view the structures as rotating 3D models, pick up the MDL Chime plugin. (4/05/98)
- Smell and Stereochemistry (Lawrence Livermore National Laboratory)
- A Web-based molecular modeling exercise that examines the differences between d-carvone and l-carvone to emphasize the correlation between molecular structure and smell. You'll need Rasmol or the Chime plugin to do the exercise.
- Smell/Olfaction Links (Lawrence Livermore National Laboratory)
- A list of technical references for further exploration of molecular theories of smell,
including many databases of olfactory receptors.
Vanillin and Eugenol
- Vanilla (UWI, Mona, Jamaica)
- The chemistry and synthesis of vanilla from eugenol, including 3D Chime models.
- Jamaican Pimento (UWI, Mona, Jamaica)
- Jamaican pimento, a source of eugenol. Includes a 3D Chime model of eugenol.
Zingerone
- Jamaican Ginger (UWI, Mona, Jamaica)
- Includes a discussion of the chemistry of ginger with a 3D Chime model of zingerone.
- Ginger (Zingiber officinale)
- About the spice.
Capsaicin
- Peppers (UWI, Mona, Jamaica)
- Pictures, gas chromatographs, and mass spectra of the Scotch Bonnet pepper, a contender for the hottest pepper on the planet. The page also includes a 3D Chime model of capsaicin.
- USCF Press Release ImagesUCSF
- Micrographs of cells before and after exposure to capsaicin show that capsaicin can cause cell damage or death.
- Hot Research on Hot Peppers (Jack Challem)
- Red Hot Receptor (Academic Press)
- Molecule Involved in Hot Pepper and Hot Bath Burning Feeling Provides New Insight into Pain (UCSF)
- The link between capsaicin burn and heat burn is described.
- Re: Chili Peppers and Capsaicin (UCSF)
- A discussion (including a JAMA reference) describing casein's ability to relieve capsaicin burns.
- Capsaicin Toxicity
- Why adding 'Pure cap' to your chili sauce isn't a good idea.
- A Word about Chiles
- Hooyoob! The reference for the Mayans military use of chiles can be found on this site, which focuses on Mayan heiroglyphics and architecture.
- The Chemical Structure of the Capsaicinoids
- Structures and properties of capsaicin, dihydrocapsaicin, nordihydrocapsaicin, homocapsaicin, homodihydrocapsaicin, ...
- HPLC methods for capsaicin determination
- How high performance liquid chromatography is used to determine capsaicin in red pepper extracts.
- Capsaicin in the Study of Pain (John Wood)
- Description and table of contents for a book by John Wood, published by Academic Press.
General Chemistry Online! Fire and Spice
Copyright © 1997-1999 by Fred Senese
Comments & questions to senese@antoine.frostburg.edu
Last Revised 12/13/99.
URL: http://antoine.fsu.umd.edu/chem/senese/101/features/capsaicin.shtml