What is involved in going from the ripe beans, to the roasted beans, to end up as a finished product?

Have you heard so much about synthetic as opposed to "natural" or "organic" chemistry and are starting to wonder whether it all happens like the right hand side of the above picture?
Then read on....
Apart from the Chemistry below, did you know that:
There are a number of varieties of both arabica and robusta.
In Jamaica, only arabica is grown and it is graded depending on the altitude at which it
is grown and processed into: lowland and high mountain. In its wild state, the
shrub grows to about 8 to 10 metres.
Although Jamaica does not have much of the world market in terms of production, the beans are well known for their exceptional quality and Blue Mountain coffee commands extremely high prices. Blue Mountain Peak stands approximately 2256 m (7402 feet) high, the average rainfall for Jamaica is about 198 cm (78 inches) and the average temperature is 27 C (82 F). This all helps in providing not only great coffee but a wonderful place to sit and watch it happen!
A brief history of coffee (from a commercial coffee supplier).
For simplicity, this will be subdivided into sections.
A range of carbohydrates, including polysaccharides and the low molecular weight sugars
(mono-, di- and trisaccharides) are found in green coffee.
Sucrose is the major free sugar present and for arabica is present at about 8% on a dry
basis.
Polysaccharides (glycans) amount to up to 50% on a dry basis of green coffee. Hydrolysis
of coffee polysaccharides has been shown to give mannose >> galactose > glucose
>> arabinose.
On roasting the coffee major changes occur, depending on the degree of roasting, e.g. from
light to dark and simple sugars such as arabinose are progressively destroyed.
These may be described in terms of three main groups of compounds: alkaloids, trigonelline together with nicotinic acid and amino acids and proteins.
Caffeine
is perhaps the best known and controversial alkaloid found in coffee and it is present at about 1-2% on a dry weight basis in arabica.Trigonelline
has received considerable attention as a nitrogen containing component of coffee.These are esters of quinic acid. For example, between caffeic acid and the 5-OH group. |
The analysis of the volatile material is usually achieved by initial separation using
Gas Chromatography or HPLC. For example, headspace analysis methods involve sampling the
vapour phase which is directly above the sample.
In the case of roasted coffee, a simulation of a GC,
in JCAMP-DX format, can be downloaded.
The following sensitive map is a simulation of a GC/MS.
For those running Win95 or WinNT who have already downloaded the new CHIME plugin (vs 2) that
incorporates our JCAMP-DX spectrum viewer, then selecting a region of the chromatogram
will download the GC and selecting a numbered box will download the Mass Spectrum (or PDB
if I can't find the MS), for that constituent.
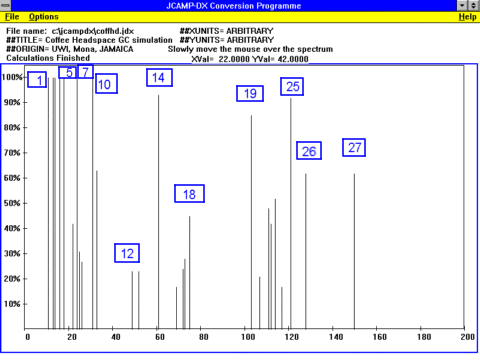
Only the more intense peaks have been shown and they have been identified as:
The numbers in brackets are the FEMA codes (Flavor and Extract Manufacturers'
Association of the USA), found in the Aldrich Flavors and Fragrances Catalog.
Most of these volatile compounds are derived from pyrolysis or from reactions occurring
during the roasting of the raw bean. These reactions involving sugars, amino acids,
organic acids and the phenolic compounds give rise to the characteristic aroma and flavour
associated with the different types of coffee. The nature of the volatile compounds and
the exact composition found is dependent on a variety of factors that include the location
during growth (eg climate and soil conditions), the storage of the beans (both during
harvesting and subsequent to roasting) and the roasting conditions used (type of
equipment, time and temperature).
An indication of the diversity of the composition of roasted coffee can be seen from
the following numbers, which highlights the sensitivity of the GC/MS detection method:
81 pyrazine containing compounds
15 pyridine derivatives
28 oxazoles have been detected and identified.
Aliphatic carboxylic acids play a large role in the quality of coffee and coffee
infusions. Changes in pH can lead to ionisation of functional groups (eg phenolic hydroxy
groups) and this can alter the flavour of the product.
A number of acids reported to be present in coffee have characteristic flavours and their
thresholds in aqueous solution may be as low as under 10 ppm. For example, 2-Methylvaleric
acid is reported to impart a flavour of cocoa or chocolate, whereas pyruvic acid gives
rise to a burnt caramel flavour.
In green coffee, non-volatile acids such as citric acid, malic acid, oxalic acid and
tartaric acid make up less than 2%.
In roasted coffee, over 30 aliphatic acids have been identified. These include 15
non-volatile monocarboxylic acids C1-C10, whilst the remainder are volatile. In general,
the darker the roast, the lower the acid content.
Reference:
To learn more about the chemistry of coffee, see:
"Coffee" Volume 1:Chemistry. Edited by R.J. Clarke and R.Macrae,
Elsevier Applied Science Publishers, London and New York, 1985.
In 1994, the Jamaica Coffee Industry suffered losses estimated at over J $ 70,000,000,
due in part, to borer infestation. The Coffee Berry Borer originated in East Africa and
was first reported in 1867. Its first appearance in the Caribbean was not reported until
1971.
It appears that endosulphan, the pesticide used to
spray the shrubs, may not be as effective as once thought and in fact, a mutant form of
the insect seems to be resistant. This new form was found in New Caledonia and has NOT yet
been seen in Jamaica.
In Jamaica, the recommended dose of endosulphan is 600-800 ml / 200 l water.
Reference:
The Gleaner, Saturday 17th February, 1996 page 4C.
The crop year is from 1st August to 31st July of the following year.
| CLEAN BEAN PRODUCT EXPORT SALES | ||||
| Blue Mtn | Lowland | Volume | Value | |
| Crop year | lbs | lbs | lbs | US $ |
| 1981/82 | 404,165 |
2,934,728 |
2,046,208 |
6,503,556 |
| 1987/88 | 1,263,730 |
3,225,880 |
2,970,000 |
9,245,801 |
| 1991/92 | 2,050,000 |
2,910,000 |
2,467,740 |
13,985,009 |
| 1995/96 | 2,572,250 |
1,907,211 |
3,052,535 |
24,296,347 |
The retail price of Blue Mountain coffee in Japan ranges from US$ 100 to $130 per kilogram compared to $20 to $40 for the blended Blue Mountain coffee. The blend is governed by Japanese regulations and must contain at least 30% of Jamaican coffee once the Blue Mountain name is used.
The figures show the increasing trend in production of Blue Mountain coffee and reveal the need for more lowland coffee. The Japanese companies are now forced to use Blue Mountain in their blends with Colombian and Brazilian coffee due to the shortage.
Reference:
The Gleaner, Sunday 8th June-97, pages 8 and 11A.
The answers
to some Frequently Asked Questions about coffee and caffeine have been collected and posted at the University of Waterloo.This site and in particular this page, was nominated for a Best of the Chemical Web
award at the American Chemical Society meeting in August 1995. For details see Chem. &
Eng. News, Nov 13, 1995, page 35-46. or The ACS WWW site
under "Whats New" or "Hot Articles".
Other references to "Chemistry on the Infobahn" can be found here, or at the
UK mirror here.
"An excellent site showing chemistry from everyday life. "
Was the description given by ChemWeb.COM when it awarded this page the ![]()
Pick for 21st November 1997.
Pick of the day, Wednesday 25th February, 1998 ![]()
Return to links to the chemistry of other Jamaican items, including spices and fruit and vegetables.
 Return to Chemistry, UWI-Mona, Home Page
Return to Chemistry, UWI-Mona, Home Page
Created Feb 1995. Last modified 12th October 1999.
URL http://wwwchem.uwimona.edu.jm:1104/lectures/coffee.html