Conversion of ethanol to gasoline range hydrocarbons using a zeolite catalyst (HZSM-5)
Introduction
The investigation of alternative and renewable sources of fuel is of special interest
to countries lacking petroleum reserves. Over the past 5 or 6 years, a project in the
Department of Chemistry has focussed on the conversion of ethanol to petrol by the use of
the synthetic zeolite HZSM-5. |
 |
A related process, which uses methanol as substrate (obtained by converting methane
from natural gas) has been developed to industrial scale in New Zealand and has the
capacity to supply about one third of their petrol requirements.
Our study has been concerned with catalyst design for the conversion of ethanol with
regard to the manufacture of liquid and gaseous fuels.
HZSM-5 can be considered as a derivative of silica and alumina, both very cheap reagents.
It acts as a solid acid since for each Al3+ substituted for Si4+ in the structure, an H+
ion is also needed to maintain the overall neutrality of the molecule.
The 3-D lattice built up, consists of channels through which certain small molecules can
pass and react inside by a process called shape selectivity. Bulky molecules can not enter
the channels and reaction of smaller molecules to larger ones is hindered by the size of
the channels, Figure 1.
This restriction means that only molecules with less than say 10 carbons are produced in
the reactions inside the catalyst.
Our results indicate total conversion of the ethanol and the liquid fuel produced is
comparable to commercial unleaded grade petrol with a good range of aromatic and aliphatic
hydrocarbons, Table 1.
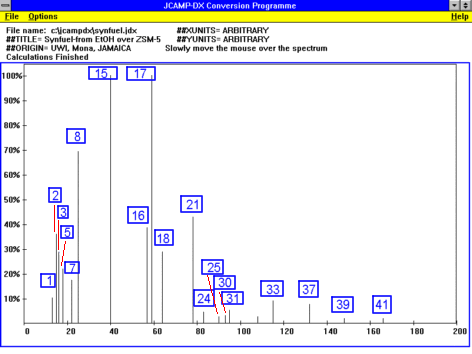
The hydrocarbons have been identified by GC and GC/MS analysis.
A simulation of a GC in JCAMP-DX format can be downloaded from here.
The major peaks have been identified as:
- 1 1-butene
- 2 2-methylpropene
- 3 2-methylbutane
- 5 4-methylpentene
- 7 methylcyclopentane
- 8 benzene
- 15 toluene
- 16 ethylbenzene
- 17 m & p-xylene
- 18 o-xylene
- 21 1-ethyl-4-methylbenzene
- 24 1,2,4-trimethylbenzene
- 25 1-methyl-4-(ethylmethyl)-benzene
- 30 1,2-diethylbenzene
- 31 1-ethyl-2,4-dimethylbenzene
- 32 2,3-dihydro-1-methyl-1-indene
- 33 naphthalene
- 37 2-methylnaphthalene
- 39 1,8-dimethylnaphthalene
- 41 2-(1-methylethyl)-naphthalene
In addition, other potential catalysts, including substituted aluminophosphates, have
been prepared and characterised by X-ray powder techniques, scanning electron microscopy,
FTIR, magic angle NMR and neutron activation analysis.
These materials have been shown to be more useful for conversion of ethanol to gaseous
products such as ethylene.
Their reactions with ethanol have significant potential for the production of
petrochemicals.
In construction
A review of these reactions and a comparison to the methanol to gasoline (MTG) process
will be given.
Table 1. Comparison of HZSM5 sample CI1/C/H and Mobil's MZIIO used in
the catalytic conversion of EtOH a.
ZSM-5 Sample* CI1/C/H MZI110/C/H
Conversion (%) >99 >99
Distribution of conversion products (wt%):
Hydrocarbons 62.8 63.4
Water 37.6 36.9
Distribution of total hydrocarbons (wt %):
Permanent gases 58.3 46.4
C5+ 41.7 53.6
Selectivity within permanent gases (wt%) :
C1 0.3 0.1
C2 9.8 10.3
C3 42.6 33.3
C4 46.9 56.3
Selectivity for aromatics in C5+ (wt%):
Nonaromatics in C5+ 29.1 40.5
Aromatics 71.9 59.5
Benzene 4.3 2.8
Toluene 21.7 11.2
Ethylbenzene 3.9 2.9
m&p-Xylene 17.4 12.5
o-Xylene 4.4 3.1
EMB** 5.8 10.7
TMB*** 1.5 3.7
2-Methylnapthalene 1.0 0.4
Others 11.9 12.2
a Reactor temperature:3750C, WHSV:6.48hr-1, N2 flow rate: 20cm3/min, wt
of catalyst: 1.0g.
*Zeolite composition: CI1/C/H-Na0.8H3.4Al4.2Si92O192.19H2O (Si/Al=21.9)
MZ110/C/H-Na0.1H2.9Al3.0Si93O192.7H2O (SiAl=26.3)
Included in the non-aromatic hydrocarbons are:
dimethyl (cyclo) pentanes, methylcyclopentane, ethylcyclopentane and di-
and trimethylbutanes, etc.
**EMB: 1-Ethyl-4-methylbenzene
***TMB: 1,2,4-trimethylbenzene
For the answers to some frequently answered questions on gasoline, oxygenates etc., see
here.
 Return to Chemistry, UWI-Mona, Home Page
Return to Chemistry, UWI-Mona, Home Page
Created Feb 1995. Last modified May 10th 1997,rjl


 Return to Chemistry, UWI-Mona, Home Page
Return to Chemistry, UWI-Mona, Home Page