Magnetic Moments
Magnetic moments are often used in conjunction with electronic spectra to gain
information about the oxidation state and stereochemistry of the central metal ion in
coordination complexes.
A template is provided for the calculations involved for the Gouy
method.
For first row transition metal ions in the free ion state, ie isolated ions in a vacuum,
all 5 of the 3d orbitals are degenerate.
If a simple crystal field theory approach is used for these ions when they are present in
octahedral complexes, then the d orbitals are no longer degenerate but are split such that
2 orbitals, the dx2-y2 and the dz2 are at higher energy than the dxy, dxz, dyz.
For ions with between 4 and 7 d electrons, this gives rise to 2 possible arrangements
called either high spin-low spin or weak field-strong field respectively. See an
interactive JAVA script for examples.
In tetrahedral complexes the orbitals are again split, such that 2 orbitals (the dx2-y2
and the dz2) are now at lower energy than the remaining 3. Tetrahedral complexes are ALL
high spin since the difference between the 2 subsets of orbitals is much smaller than is
found in octahedral complexes (delta tet=4/9 delta oct).
The formulae most frequently used to calculate magnetic moments of first row transition
metal ion complexes are given below:
mSO = SQRT(4S(S+1))
mS+1 = SQRT(4S(S+1)+L(L+1))
meff = mSO (1 - al/D )
The first formula is known as the spin only formula, the second takes into account
possible contributions from orbital angular momentum and the third is used to make small
adjustments when the ground term for the metal ion is either A or E.
S is the spin quantum number = 1/2 for each unpaired electron
L is the orbital angular momentum
alpha is a constant = 2 for E and 4 for A ground terms
lambda is the spin orbit coupling constant
delta is the crystal field splitting parameter found from the spectrum
of the complex.
To illustrate:
predict the variation of the magnetic moments for the series of tetrahedral complexes
Copyr2Cl2, Copyr2Br2 and Copyr2I2.
Their visible spectra have been recorded in chloroform
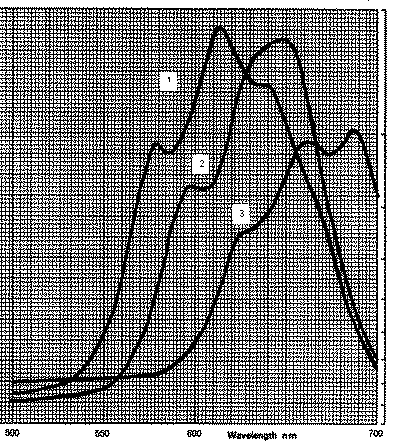
and from the centre of their main absorption bands in the visible region, lambda can be
seen to be roughly:
1 Copyr2Cl2 615nm
2 Copyr2Br2 630nm
3 CopyrI2 670nm
These peaks represent the third transition expected for these complexes, the other
bands occur in the infrared region, so although we may be able to place the halides into
order of the spectrochemical series, we need to consult an Orgel diagram before trying to
calculate delta.
The Orgel diagram appropriate for these types of complexes is given below:
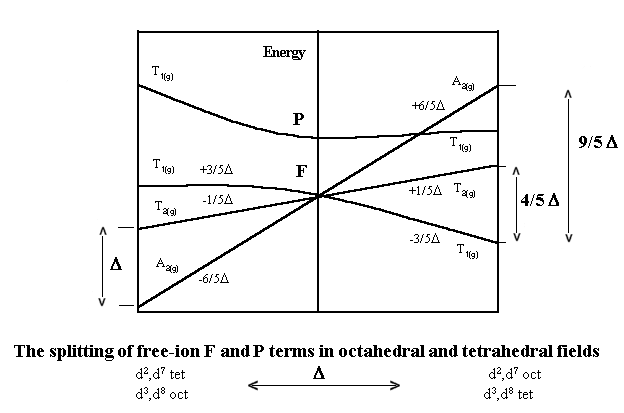
and for d7 tetrahedral the ground term is 4A2.
Three peaks are predicted in their electronic spectra, namely:
T2 - A2
T1(F) - A2
T1(P) - A2 ** the band observed in the visible region
The energy of these third transitions can be taken to be approximately 6/5 delta + 15B
(ignoring configuration interactions and where B is the Racah parameter). For Co(II)
tetrahedral complexes B has generally been found to be about 750 cm-1.
Hence delta for the three complexes above can be calculated to be roughly:
Copyr2Cl2 (16260-15·750) x 5/6 = 4175 cm-1
Copyr2Br2 (15870-15·750) x 5/6 = 3850 cm-1
Copyr2I2 (14925-15·750) x 5/6 = 3063 cm-1
The spin only magnetic moment is predicted to be 3.87 BM.
Using a value for the free ion spin orbit coupling constant of -172 cm-1 then a
better approximation can be obtained by using the third formula above. This would give a
value of:
Copyr2Cl2 3.87 (1+ 688/4175) = 4.49 B.M. found 4.42 BM
Copyr2Br2 3.87 (1+ 688/3850) = 4.57 B.M. found 4.50 BM
Copyr2I2 3.87 (1+ 688/3063) = 4.76 B.M. found 4.48 BM
In the case of the series;
CoI42-, CoBr42-, CoCl42- Co(NCS)42-
the magnetic moments have been recorded as
4.77 4.65 4.59 4.40 BM
showing even more clearly the inverse effect of the spectrochemical series on the
magnetic moment.
Created and maintained by Dr. Robert J. Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created Dec 1995. Last modified 18th April-98.
URL http://wwwchem.uwimona.edu.jm:1104/spectra/MagMom.html

