| The handling of carbon dioxide
|
The body produces CO2 at a rate of 200-800 mL/min. As described above, the CO2 dissolves in water and generates carbonic acid, which in turn dissociates into hydrogen and bicarbonate ions:
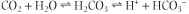 Thus, CO2 generates acid (large amounts of hydrogen ion).
Thus, CO2 generates acid (large amounts of hydrogen ion).
|
| Erythrocytes play an important role in the transport of CO2 between the tissues, and the lungs
|
| ANEMIA IS A CAUSE OF SHORTNESS OF BREATH AND TIREDNESS |
| A 35-year-old man presented with shortness of breath after climbing two flights of stairs. His chest radiograph was normal, as were examination of the heart and the electrocardiogram; blood gases were normal. He was taking a non-steroidal anti-inflammatory agent (NSAID) for joint pain. His blood cell count revealed a hemoglobin value of 10 g/dL (reference range for men 13-18 g/dL) and a reduced mean corpuscular volume of 72 dL (reference range 80-100). Serum ferritin concentration was low at 10 ug/L (reference range 30-280). |
| Comment. This person had chronic iron deficiency anaemia. Anemia can present with general symptoms such as tiredness or breathlessness. The patient had a gastric ulcer caused by the NSAID with increased loss of blood from the gastrointestinal tract. |
| We already know that in plasma, the above reaction is nonenzymatic and proceeds slowly, generating very small amounts of carbonic acid, which remain in equilibrium with a substantial amount of dissolved CO2. The same, but much faster, reaction occurs in the erythrocytes. Here the reaction is catalyzed by the carbonic anhydrase, which 'fixes' CO2 as
bicarbonate. The hydrogen ion resulting from the dissociation of carbonic acid is buffered by hemoglobin.
|
| The bicarbonate produced by carbonic anhydrase in the erythrocytes moves to plasma in exchange for the chloride ion (this is known as 'chloride shift') (Fig. 23.6). Approximately 70% of all CO2 produced in tissues becomes bicarbonate; approximately 20% is carried as carbamino groups on hemoglobin molecules; and only 10% travels simply dissolved in plasma.
|
| In the lungs, the dissolved CO2 diffuses into the alveolar space
|
| In the lungs, the increased pO2 facilitates the dissociation of CO2 from hemoglobin. This is known as the Haldane effect. In parallel, hemoglobin releases its hydrogen ion, which reacts with bicarbonate and forms carbonic acid which then releases CO2.
|
| page 339 |  | | page 340 |
| Figure 23.6 Erythrocyte role in CO2 transport. Most of the CO2 produced in tissues is converted to bicarbonate for transport to the lungs: approximately 20% of the total amount is transported bound to hemoglobin as carbamino groups, and small amounts are transported as a dissolved gas in plasma. |
|