| Caspases are involved in apoptosis
|
| page 590 |  | | page 591 |
|
Table 41-1.
Relationship between various cancers and stages of the cell cycle. |
| Body_ID: None |
| Cancer and the cell cycle |
| Body_ID: T041001.50 |
| Cancer | Cell cycle element | Outcome: status of the retinoblastoma protein (Rb) |
| Body_ID: T041001.100 |
| | cyclin expression increased as a result of gene amplification or chromosomal translocation | hyperphosphorylation and inactivation of retinoblastoma protein (Rb), leading to deregulated malignant cell proliferation |
| Body_ID: T041001.150 |
| breast cancer, leukemia testicular carcinomas breast tumors | cyclin D1
cyclin D2
cyclin E | |
| Body_ID: T041001.200 |
| retinoblastomas small-cell lung carcinomas osteosarcomas | mutational inactivation of Rb genes | loss of Rb control of cell cycle, leading to deregulated malignant cell proliferation |
| Body_ID: T041001.250 |
| cervical carcinomas | sequestration of Rb by the human papilloma virus E7 protein | loss of Rb control of cell cycle |
| Body_ID: T041001.300 |
| variety of tumor cells | deletion of CDKI genes, p14, p15 | loss of inhibition of cyclin D-CDK4/6 complexes, resulting in inappropriate hyperphosphorylation and inactivation of Rb |
| Body_ID: T041001.350 |
| melanomas | mutant CDK4 demonstrating resistance to CDKI gene products, p14, p15 | loss of inhibition of cyclin D-CDK4/6 complexes, resulting in loss of Rb control of cell cycle |
| Body_ID: T041001.400 |
|
| Body_ID: T041001.450 |
Some degree of disruption of the cell cycle machinery is likely to occur in every type of cancer cell (Table 41.2). As most proliferative decisions are made at the restriction point in late G1-phase, the key target for cellular transformation appears to be the retinoblastoma protein, Rb. Indeed, it is now clear that the signal transduction events leading to Rb phosphorylation and functional inactivation are disrupted in many cancer cells, suggesting that Rb inactivation may be crucial to deregulated, malignant cell proliferation.
|
| Disruption of the apoptotic machinery in B cell leukemias |
| During lymphopoiesis, B (75%) and T (95%) cell progenitors that fail to rearrange their antigen receptor genes in a productive, non-self manner are programmed to die by apoptosis. The finding that the cell survival gene, Bcl-2, was subject to a chromosomal translocation in human follicular lymphomas suggested that subversion of apoptotic pathways may have a key role in malignant transformation. Thus, by deregulating the key antiapoptotic protein, Bcl-2, this translocation promotes the survival of cells that would otherwise die, and which then acquire the additional mutations required for malignant transformation. |
| Bcl-2 has now been shown to belong to a family of proteins that modulate caspase activity and hence are intimately involved in the regulation of selection of the alternative pathways of cell survival or apoptosis. Thus, whereas family members such as Bcl-2 and Bcl-xL inhibit apoptosis, their cell survival activities can be antagonized by Bcl-xS (a lower-molecular-weight splice variant of Bcl-xL), Bak, Bax, and Bad. The antagonistic actions of these proteins can be illustrated by studies showing that, whereas loss of Bax promotes tumor formation in mice, overexpression of Bax can suppress cellular transformation in vitro. Similarly, a number of viruses (e.g. adenovirus, Epstein-Barr virus, African swine fever virus, herpes virus Saimiri, and human herpes virus 8) enhance their replication by encoding viral homologs of Bcl-2 to promote survival of the host cell. Finally, the tumor suppressor, p53, appears to activate apoptosis, at least in part, by downregulating the expression of Bcl-2 and upregulating that of Bax; this provides an additional rationale for p53 being the most commonly mutated gene in human cancer. |
The caspases act by cleaving key target proteins, resulting in the systematic disassembly of the apoptotic cell by:
- halting cell cycle progression;
- disabling homeostatic and repair mechanisms;
- initiating the detachment of the cell from its surrounding tissue structures;
- dismantling structural components such as the cytoskeleton;
- flagging the dying cell for phagocytosis.
|
| Overexpression of active caspases is sufficient to cause cellular apoptosis.
|
| page 591 |  | | page 592 |
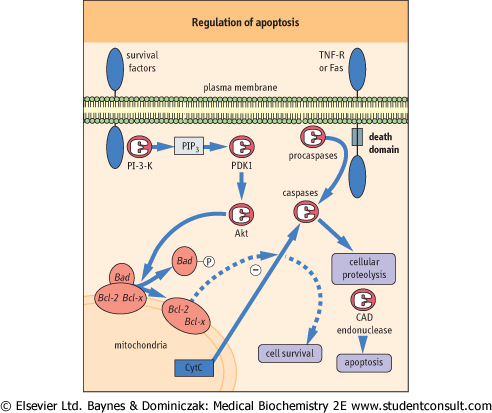
|
| Figure 41.7 Regulation of apotosis and growth factor-mediated cell rescue. Cell death receptors, such as TNF receptor (TNF-R) or Fas, recruit procaspases such as caspase 8 to their 'death domain' transducing molecules (FADD, TRADD, RIP, and RAIDD), thereby inducing the proteolytic caspase activation cascade of apoptotic cell death. In contrast, the Bcl-2 family-regulated cascade may be modulated by survival factors coupled to phosphoinositide-3-kinase (PI-3-K), leading to rescue of cells from apoptosis and promotion of cell survival. PI-3-K catalyses the production of phosphatidylinositol 3,4,5-trisphosphate (PIP3), which stimulates a PIP3-dependent kinase (PDK), resulting in phosphorylation and activation of the protein kinase, Akt. In turn, Akt phosphorylates the proapoptotic Bcl-2 family member, Bad, inducing dissociation of Bad from heterodimeric complexes with Bcl-2 or Bcl-xL and allowing Bcl-2/Bcl-xL to antagonize cell death by preventing release of cytochrome c (Cyt c) and caspase activation. CAD, caspase-dependent endonuclase. |
| ANALYSIS OF CELL CYCLE PROGRESSION AND APOPTOTIC CELLS |
| The DNA content of cells varies through the cell cycle, increasing during S phase from diploid chromosomal DNA in G0/G1 to tetraploid at G2/M, before returning to diploid again after M phase. Furthermore, subdiploid DNA content is an indicator of apoptosis. The cell cycle status of cells can therefore be assessed in the laboratory by measuring DNA content. Flow cytometry, which measures the fluorescence of individual cells, can be combined with a variety of fluorescent staining techniques to assess the proportions of cells in a population that are at the different stages of the cell cycle or undergoing apoptosis. |
| For example, staining with propidium iodide (PI), which intercalates into the DNA helix, measures the total DNA content of cells; 4'-6'-diamidino-phenylindole-2HCl (DAPI) works in a similar manner by preferentially binding to A-T base pairs. Incorporation of bromodeoxyuridine (BrdU), an analog of the DNA precursor thymidine, into the newly synthesised DNA of cells progressing through S phase can also be detected by flow cytometry using anti-BrdU antibodies conjugated to fluorescent dyes. |
| Non-fluorescent carboxy-fluoresceindiacetate succinimidyl ester (CFDA SE), which is converted to fluorescent CFSE by the action of cytoplasmic esterases, is a very effective reagent for studying the division of proliferating cells. When CFSE-stained cells divide, CFSE is uniformly distributed between daughter cells; each division reduces the CFSE fluorescence intensity of daughter cells by approximately half. Flow cytometric analysis therefore reveals discrete frequency distributions of cells with progressively reduced fluorescence levels (histogram peaks), representing successive generations of cells (Fig. 41.8). |
| Multi-color staining using fluorescent dyes with different emission spectra can also be performed to yield extra information about cell subpopulations. For example, antibodies against cell-specific markers conjugated to other fluorescent dyes can be employed to establish whether an agent causes all cells or an individual cell type within a mixed cell population to proceed through the cell cycle, arrest in G0 phase, or undergo apoptosis. |
 |
| Caspases are expressed as inactive procaspases, and it is likely that caspase activation occurs in a cascade fashion, with the initial activation of a regulatory caspase serving to activate downstream effector caspases by proteolysis. Cell death
receptors, such as tumor necrosis factor (TNF)-R and Fas (also known as CD95 or APO-1), mediate apoptotic cell death pathways in a number of cell types but particularly in cells of the immune system. They initiate apoptosis by directly recruiting procaspases, such as caspase 8, to their accessory 'death domain' transducing molecules thereby inducing the proteolytic caspase activation cascade (Fig. 41.7). These death domain molecules include Fas-associated death domain protein (FADD), TNF-receptor associated death domain protein (TRADD), receptor interacting protein (RIP), and RIP-associated ICE-like protease with a death domain (RAIDD). The precise molecular mechanisms of effector caspase activation are as yet unknown. However, it has recently
emerged that proteolysis and activation of effector caspases is facilitated by binding to cytochrome c, which is released from the mitochondria during the process of apoptosis.
|
|