The Kirkvine works were completed around 1952 and the first shipment of alumina was consigned to a Norwegian smelter in January 1953.
The first commercial extraction of alumina (Al2O3) from bauxite has been attributed to Henri Sainte-Claire Deville in about 1854.
Soon after this, in 1888, Karl Joseph Bayer described what is now known as the Bayer Process, which led to a dramatic reduction in the cost of aluminum metal. Today, it is an everyday commodity, rather than a precious metal.
Although deposits of aluminous red earth have been known to occur in the Tertiary Limestone areas (which covers two thirds of the land surface of Jamaica) since the 1820's, it was not until the 1940's that their economic significance as an ore of aluminum was recognised.
In October, 1943, Alcan was incorporated under the name Jamaica Bauxites Limited as a
Jamaican company to investigate the commercial potential of Jamaican bauxite. In the same
year, 2500 tonnes of ore was shipped to the USA for process investigation and it was
realised that the bauxite was suitable for processing using Bayer technology.
The Kirkvine works were completed around 1952 and the first shipment of alumina was
consigned to a Norwegian smelter in January 1953.
A Bayer plant in Jamaica
The Bayer Process, which continues to be the most economical method of manufacturing
alumina can be schematically summarised in a flow chart,
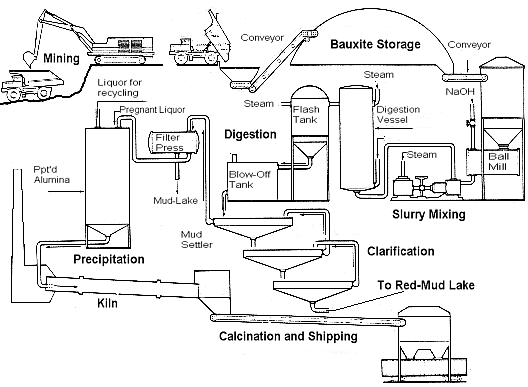
and involves the following operations:
Immediately prior to mining any deposit, the land is cleared and the top soil, to a minimum of 6 inches, removed and stockpiled for later replacement when mining is completed.
The surface occurrence of the ore (usually less than 100 feet) makes the deposits suitable for mining by simple opencast methods. Due to the soft, earthy nature of the ore, no drilling or blasting is generally required.
Deposits are located in areas varying from gentle undulating to rugged, hilly terrain involving major capital expenditures in establishing a system of ore transportation.
Mining areas in Jamaica
The exact procedure required for digestion, depends on the nature of the ore deposits.
The following Table outlines a number of minerals commonly found in bauxites:
Gibbsite (hydrargillite) alpha-Al2O3·3H2O Boehmite alpha-Al2O3·H2O Diaspore beta-Al2O3·H2O Hematite alpha-Fe2O3 Goethite alpha-FeOOH Magnetite Fe3O4 Siderite FeCO3 Ilmenite FeTiO3 Anatase TiO2 Rutile TiO2 Brookite TiO2 Halloysite Al2O3·2SiO2·3H2O Kaolinite Al2O3·2SiO2·2H2O Quartz SiO2
Jamaican bauxite is mainly gibbsitic but some amount of boehmite is also present. The average grade of bauxite mined is of the order of 45% available alumina and 1.5% reactive silica.
In order to remove the iron oxides and most of the silicon oxides present, the ore is first treated with sodium hydroxide. The digestion process takes advantage of the solubility of amphoteric aluminum oxides to form a solution of aluminate ions, whilst the basic iron oxides which form do not dissolve and are separated by filtration.
Gibbsite Al2O3·3H2O + 2NaOH ---> 2 NaAlO2 + 4 H2O (135°-150° C)
Boehmiite Al2O3·H2O + 2NaOH ---> 2 NaAlO2 + 2 H2O (205°-245° C)
Diaspore Al2O3·H2O + 2NaOH ---> 2 NaAlO2 + 2 H2O (high T° and P)
Complete extraction from diasporic bauxite requires stronger caustic solutions, in addition to higher temperatures and pressures. In general the reaction equilibria above move to the right with increases in caustic soda concentration and temperature. In practice this means that for deposits containing the more easily recovered Gibbsite only, production costs are much lower than when Boehmite or Diaspore are present.
The control of silica in the conventional Bayer Process is most important and in fact ores having reactive silica greater than 7% cannot be economically processed.
Unlike quartz, which is considered virtually non-reactive at Gibbsite extraction temperatures, some minerals, including kaolins, dissolve rapidly and the reaction of the silica can give rise to appreciable loss of caustic soda and aluminum.
The control of silica is generally carried out during, or prior to, the digestion step, and generally involves dissolution, eg for kaolin
Al2O3·2SiO2 + NaOH ---> NasSiO3
and desilication via precipitation.
Na2SiO3 + NaAlO2 ---> Na2O·Al2O3·2SiO2
Dissolution is necessary to supersaturate the liquid to a point where the sodalite formed acts as a seed to precipitate more sodalite. The rate of precipitation is found to increase with temperature, however at 135°-150° C it is significantly slower than is required for complete Gibbsite extraction which occurs within minutes. The need for desilication therefore means that the material must be held at the digestion temperature long enough to allow the silica to precipitate.
Still to come

and the latest ideas on dry stacking
References.
see for example,
R.J. Lancashire, "Bauxite and aluminium production"
Education in Chemistry, May 1982, pps 62-64.
For information on Bayer Process research see the CSIRO Division of Mineral Products.
Further information is available from Nabalco (a bauxite and alumina mining company).
For the latest statistics on bauxite/alumina production check the US Geological Surveys site
The Jamaica Bauxite Institute has released figures for the first quarter of 1997 which
suggest that this year will be a record high for total bauxite production.
Total bauxite production is measured as crude bauxite plus bauxite converted into alumina
and it has reached 3 million tonnes for the first quarter this year (1997). This compares
with a 11 million for 1995, 11.7 million tonnes in 1996.
The alumina plants are said to be working at 96% capacity for the first time in many years
and for the first quarter produced 816,999 tonnes.
Photos featuring bauxite/alumina production can be downloaded from Alumax Inc.
A description of the Hall process for electrolytic reduction of alumina to aluminum is appropriately provided at Oberlin College, from where Charles Hall had graduated only eight months prior to his successful experiment.
Home
Ja themes Lab. Manuals Lectures Search Software Spectra Staff Survey Tutorials Weekly Message UWI-Mona HomeCreated Feb 1995. Last modified 12th September-97.
URL http://wwwchem.uwimona.edu.jm:1104/lectures/bauxite.html