| Myoglobin: an O2-storage protein
|
Located in the cytosol of muscle cells, Mb binds O2 that has been released by Hb in the tissue capillaries and has subsequently diffused across cellular membranes. This stored O2 is readily available to organelles, particularly the mitochondrion, that carry out oxidative metabolism. With its single ligand-binding site, the reversible reaction of Mb with O2
 may be described by the following equations,
may be described by the following equations,
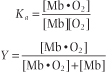 in which Ka is an affinity or equilibrium constant and Y is the fractional O2 saturation. Combining these two equations, expressing the concentration of O2 in terms of its partial pressure pO2, and substituting the term P50 for 1/Ka yields the equation for the O2 saturation curve of Mb:
in which Ka is an affinity or equilibrium constant and Y is the fractional O2 saturation. Combining these two equations, expressing the concentration of O2 in terms of its partial pressure pO2, and substituting the term P50 for 1/Ka yields the equation for the O2 saturation curve of Mb:
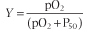
|
| By definition, the constant P50 is the value of pO2 at which Y = 0.5 or half the ligand sites are occupied (saturated by O2). In a plot of Y versus pO2, the equation for ligand binding by Mb describes a hyperbola (Fig. 4.3) with P50 ≈ 4 mm Hg. The low value of P50 reflects a high affinity for O2. In the capillary beds of muscle tissues, pO2 values are in the range of 20-40 mm Hg. Predictably, working muscles exhibit lower pO2 values than muscles at rest. With its high affinity for O2, myocyte Mb readily becomes saturated with O2 that has entered from the blood. As O2 is consumed during aerobic metabolism, O2 dissociates from Mb and diffuses into mitochondria, the power plants of the muscle cell.
|
|