| Interactions with allosteric effectors
|
| Allosteric proteins and effectors
|
| Pulse oximetry ('pulse-ox') is a non-invasive method of estimating the oxygen saturation of arterial Hb. Two physical principles are involved: first, the visible (650-660 nm) and infrared (850-940 nm) spectral characteristics of oxy- and deoxy-Hb are different; second, arterial blood flow has a pulsatile component that results from volume changes with each heart beat. Therefore, transmission or reflectance measurements are made in a translucent tissue site with reasonable blood flow, commonly a finger, toe, or ear of adults and children, or a foot or hand of infants. The pulse oximeter's photodetector and microprocessor calculate an oxygen saturation (SpO2) that typically correlates within 4-6% of the value found by arterial blood gas analysis. Pulse oximetry is used to monitor the cardiopulmonary status during local and general anesthesia, in intensive care and neonatal units, and during patient transport. Body movement, radiated ambient light, elevated bilirubin, artificial or painted fingernails can interfere with pulse oximetry. |
| page 40 |  | | page 41 |
| Figure 4.6 Allosteric effectors decrease the oxygen affinity of hemoglobin. O2 interaction with hemoglobin is regulated by allosteric effectors. Under physiologic conditions HbA exhibits a highly cooperative O2 saturation curve. With an increase in the erythrocyte concentration of any of three allosteric effectors, H+, CO2, or 2,3-bisphosphoglycerate (2,3-BPG), the curve shifts to the right (position B), indicating a decreased affinity for O2 (increase in the P50 value). Actions of the effectors that modulate O2 affinity appear to be additive. Conversely, a decrease in any of the allosteric effectors shifts the curve to the left (position A). Increasing temperature will also shift the curve to the right. The sensitivity of O2 saturation to [H+] is known as the Bohr effect. Normal ranges of O2 measured in pulmonary and peripheral tissue capillaries are indicated by shaded areas. |
| Hb is one of the best-studied examples of an allosteric protein, a protein that exhibits changes in ligand (or substrate) affinity under the influence of small molecules. These small
molecules, called allosteric (meaning other place or site) effectors, bind to proteins at sites that are spatially distinct from the ligand-binding sites. Through long-range conformational effects, they alter the ligand or substrate binding affinity of the protein. Allosteric proteins are typically multisubunit proteins. An allosteric effector may exert either a positive influence on ligand interaction (increased affinity) or a negative influence (decreased affinity). The O2 binding affinity of hemoglobin is affected positively by O2 (above), and also also by a number of chemically different allosteric effectors, including H+, CO2, and 2,3-bisphosphoglycerate (2,3-BPG) (Fig. 4.6). When an allosteric effector affects its own binding to the protein (at other sites), the process is termed homotropic, e.g. the effect of binding of O2 at one site on hemoglobin enhances the affinity for binding of O2 to other sites on hemoglobin. When the allosteric effector is different from the ligand whose binding is altered, the process is termed heterotropic, e.g. the effect of H+ on the P50 for oxygen binding to hemoglobin.
|
| The O2 affinity of Hb is exquisitely sensitive to pH, a phenomenon called the Bohr effect. It is most readily described as a right shift in the O2 saturation curve with decreasing pH. Thus, an increased concentration of H+ (decreased pH) favors an increased P50 (lower affinity) for O2 binding to hemoglobin. To understand the Bohr effect at the level of protein structure and to appreciate the role of H+ as an allosteric effector, it is important to recall that Hb is a highly charged molecule. Experimental evidence suggests that the residues that participate in the Bohr effect are the N-terminal amino group of the α-chains and the side chains of His122 α and His143 β. The pK values of these residues differ sufficiently between the deoxygenated and oxygenated forms of Hb to cause the uptake of about two H+ by the T-structure (deoxygenated Hb). These interactions, in turn, are linked directly to the decrease in O2 affinity of the T-state of Hb.
|
| When Hb binds O2, protons dissociate from the weak acid functions. Conversely, in acidic media, protonation of the conjugate bases inhibits O2 binding. During their circulation between pulmonary alveoli and peripheral tissue capillaries, erythrocytes encounter markedly different conditions of pO2 and pH. The high pO2 in the lungs promotes ligand saturation, yet it also forces protons from the Hb molecule to stabilize the R-state. In the capillary bed, particularly in metabolically active tissues, the pH is slightly lower, due to the production of acidic metabolites, such as lactate. Oxygenated Hb, upon entering this environment, will acquire some 'excess' protons and shift toward the T-state, promoting release of O2 for uptake in tissues for aerobic metabolism (Chapter 23).
|
| Effects of CO2 and temperature
|
Closely related to the Bohr effect is the ability of CO2 to alter the O2 affinity of Hb. Like the negative allosteric effect of H+, the increase in pCO2 in venous capillaries decreases the affinity of hemoglobin for O2. Accordingly, a right shift in the ligand saturation curve occurs as pCO2 increases. It should be emphasized that the allosteric effector is, in fact, CO2, not HCO3-. CO2 reacts reversibly with the unprotonated N-terminal amino groups of the globin polypeptides to form carbamino-Hb:
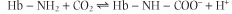
|
| This transient chemical modification of Hb is not only a specialized example of allosteric control, resulting in a stabilization of deoxygenated Hb; it also represents one form of transport of CO2 to the lungs for clearance from the body.
|
| page 41 |  | | page 42 |
| ACUTE MOUNTAIN SICKNESS (TOO HIGH TOO FAST) |
| Acute mountain sickness (AMS) develops in 20-25% of individuals who ascend rapidly to altitudes above 2500- 4000 m and in the majority of those who climb even higher. Symptoms include shortness of breath, rapid heart rate, headache, nausea, anorexia, and sleep disturbance. AMS is usually benign; it occurs with greater frequency in the young. The most severe form is high-altitude cerebral edema (HACE), a potentially life-threatening condition that is characterized by ataxia and other neuromuscular and neurological problems. HACE has been proposed to be a hypoxia-induced, vasogenic edema whose pathophysiology involves altered permeability of the blood-brain barrier and imbalance among fluid compartments in the central nervous system. |
| At 4000 m the barometric pressure is 460 torr, leading to an ambient partial pressure of O2 of 96 torr (sea level, 159). Physiological calculations yield values of tracheal pO2 = 86 torr (sea level, 149), alveolar pO2 = 50 torr (sea level, 105), and arterial pO2 = 45 torr (sea level, 100). At this arterial partial pressure of O2, hemoglobin saturation is only 81% (see Fig. 4.3). Consequently, the O2 content of arterial blood decreases to ∼16.0 mL/L (sea level, 19.5), based on an Hb concentration of 150 g/L. Decreased tissue O2 can potentially disrupt cellular metabolism. Humans adapt to high altitude (acclimatization) by several mechanisms. Hyperventilation is a critical short-term response that serves to decrease alveolar pCO2 and, in turn, increase alveolar pO2. Arterial pH is also increased during hyperventilation, leading to a higher affinity of Hb for O2. A gradual increase in 2,3-bisphosphoglycerate also occurs in response to chronic hypoxia. Another important adaptative mechanism is polycythemia, an increase in erythrocyte concentration that results from erythropoietin stimulation of bone marrow cells. Within 1 week of acclimatization the Hb concentration can increase by as much as 20% (30 g/L) to provide near-normal arterial O2 content. |
There is a strong physiologic correlation between pCO2 and O2 affinity. CO2 is a major product of mitochondrial oxidation and, like H+, will be particularly abundant in metabolically active tissues. Upon diffusing into the blood, a small portion of CO2 reacts with oxygenated Hb, shifts the equilibrium toward the T-state, and thereby promotes the dissociation of bound O2 (Fig. 4.6). The vast majority of peripheral-tissue CO2, however, is hydrated in the presence of erythrocyte carbonic anhydrase to carbonic acid (H2CO3), a weak acid that dissociates partially to H+ and HCO3- (see Chapter 23):
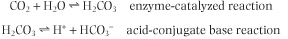
|
| Interestingly, from both the carbamination reaction and hydration/dissociation reactions involving CO2, an additional pool of protons is generated, protons that become available to participate in the Bohr effect and facilitate O2-CO2 exchange. During its return to the lungs, blood transports two forms of CO2: carbamino-Hb and the H2CO3/HCO3- acid-conjugate base pair. Blood and Hb are now exposed to a low pCO2, and through mass action the carbamination reaction is reversed and binding of O2 is again favored. Similarly, in the pulmonary capillaries, erythrocyte carbonic anhydrase converts H2CO3 into CO2 and H2O, products whose gaseous forms are expelled into the atmosphere, as discussed in Chapter 23.
|
| Working muscles not only produce the allosteric effectors H+ and CO2 as byproducts of aerobic metabolism, but they liberate heat as well. Because the binding of O2 to heme is an exothermic process, the O2 affinity of Hb decreases with increasing temperature. Thus, the microenvironment of an exercising muscle favors a more efficient release of Hb-bound O2 to the surrounding tissue.
|
| Effect of 2,3-bisphosphoglycerate
|
| An organic phosphate compound, 2,3-bisphosphoglycerate, is another important modulator of the O2 affinity of hemoglobin. A side product in the glycolytic pathway (Chapter 11), this molecule is synthesized in human erythrocytes. Like H+ and CO2, it is an indispensable negative allosteric effector that, when bound to Hb, causes a marked increase in P50. Indeed, if it were not for the high erythrocyte concentration of 2,3-BPG (∼4.1 mmol/L [1.1 g/L], nearly equal to that of Hb), the O2 saturation curve of Hb would approach that of Mb!
|
At one end of the two-fold symmetry axis within the quaternary structure of Hb there is a shallow cleft defined by cationic amino acids of the juxtaposed β subunits (Fig. 4.7). One molecule of 2,3-BPG binds to this site. A critical consequence of the conformational differences between the T- and R-states is that only deoxygenated Hb interacts with the negatively charged 2,3-BPG. Electrostatic interactions stabilize the complex between the effector and Hb. The cleft is too narrow in oxygenated Hb to accommodate 2,3-BPG. of the juxtaposed β subunits (Fig. 4.7). One molecule of 2,3-BPG binds to this site. A critical consequence of the conformational differences between the T- and R-states is that only deoxygenated Hb interacts with the negatively charged 2,3-BPG. Electrostatic interactions stabilize the complex between the effector and Hb. The cleft is too narrow in oxygenated Hb to accommodate 2,3-BPG.
|
| The importance of 2,3-BPG as an allosteric effector is underscored by observations that its concentration in the erythrocyte changes in response to various physiologic and pathologic conditions. During chronic hypoxia (decreased pO2) because of pulmonary disease, anemia or shock, the level of 2,3-BPG increases. Such compensatory increases have also been described in cigarette smokers and on adaptation to high altitudes. The net result is a greater stabilization of the deoxygenated, low-affinity T-state and a further shift of the saturation curve to the right, thereby facilitating release of more O2 to the hypoxic tissues. Under most circumstances, the rightward shift has an insignificant effect on the O2 saturation of Hb in the lungs.
|
| page 42 |  | | page 43 |
| Figure 4.7 2,3-Bisphosphoglycerate binding to deoxygenated hemoglobin. On the surface of the deoxygenated Hb tetramer where the two β-globin (purple) interact, there is a cleft formed by the N-terminal amino acid residue (Val1 β) and the side chains of His2 β, Lys82 β, and His143 β (stick models). This site consists of eight cationic groups, sufficient to bind with high affinity one molecule of 2,3-BPG (ball-and-stick model; phosphorus, orange), a molecule with five anionic groups at physiological pH. This array of positive charges does not exist in oxygenated Hb. In fetal Hb (HbF) His143 β is replaced by a Ser residue. |
|