During fasting and starvation, when hepatic glycogen is depleted, gluconeogenesis is essential for maintenance of blood glucose homeostasis. Unlike glycogenolysis, which can be turned on rapidly in response to hormonal stimulation, gluconeogenesis is a slower response, reaching maximal activity over a period of hours (Fig. 12.1); it becomes the primary source of our blood glucose homeostasis. Unlike glycogenolysis, which can be turned on rapidly in response to hormonal stimulation, gluconeogenesis is a slower response, reaching maximal activity over a period of hours (Fig. 12.1); it becomes the primary source of our blood glucose concentration about 8 hours into the post-absorbtive state (see also Chapter 20). concentration about 8 hours into the post-absorbtive state (see also Chapter 20).
|
| page 167 |  | | page 168 |
| EXCESS ALCOHOL CONSUMPTION CAN LEAD TO HYPOGLYCEMIA |
A middle-aged, emaciated, chronic alcoholic man collapsed in a bar at about 11 a.m., and was transported to the emergency room by ambulance. Another patron noted that the man had had only a few shots of vodka, and did not appear to be unusually drunk, although he was a little confused, at the time that he fainted. The bartender suggested that the man might have had a heart attack. Physical examination revealed a somewhat clammy skin, unusual for a winter morning, rapid breathing, and a rapid heartbeat. Laboratory tests indicated a blood glucose of 2.5 mmol/L (50 mg/dL), in the hypoglycemic range, and a blood alcohol level of 0.2%, suggesting intoxication. Subsequent tests indicated a normal level of creatine phosphokinase, an enzyme measured for early diagnosis of myocardial infarction, high serum aspartate aminotransferase activity, indicative of ongoing liver damage, a slightly acidic blood pH (7.29 versus normal 7.35), low pCO2, and high blood lactate. The man responded to an infusion of a glucose of 2.5 mmol/L (50 mg/dL), in the hypoglycemic range, and a blood alcohol level of 0.2%, suggesting intoxication. Subsequent tests indicated a normal level of creatine phosphokinase, an enzyme measured for early diagnosis of myocardial infarction, high serum aspartate aminotransferase activity, indicative of ongoing liver damage, a slightly acidic blood pH (7.29 versus normal 7.35), low pCO2, and high blood lactate. The man responded to an infusion of a glucose solution, regained consciousness, had brunch, and a few hours later, after a miraculous recovery, was referred to a counselor for treatment. What happened? solution, regained consciousness, had brunch, and a few hours later, after a miraculous recovery, was referred to a counselor for treatment. What happened? |
Comment. This patient probably had not eaten breakfast before starting his morning binge. His glycogen stores were negligible, so he was dependent on gluconeogenesis for maintenance of blood glucose concentration, but gluconeogenesis may be compromised both by liver disease (cirrhosis) and by the limited muscle mass available to mobilize amino acids concentration, but gluconeogenesis may be compromised both by liver disease (cirrhosis) and by the limited muscle mass available to mobilize amino acids for gluconeogenesis. The consumption of alcohol places additional stress on gluconeogenesis, since alcohol is metabolized primarily in the liver. The two-step metabolism of alcohol is relatively unregulated, leading to a rapid increase in hepatic NADH: for gluconeogenesis. The consumption of alcohol places additional stress on gluconeogenesis, since alcohol is metabolized primarily in the liver. The two-step metabolism of alcohol is relatively unregulated, leading to a rapid increase in hepatic NADH:
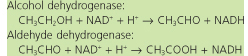 |
The increase in hepatic NADH shifts the equilibrium of the LDH reaction toward lactate, limiting gluconeogenesis from pyruvate derived from lactate (or alanine), leading to accumulation of lactic acid in blood (lacticacidemia). It also shifts cytosolic oxaloacetate toward malate, reducing gluconeogenesis from citric acid cycle intermediates, and shifts dihydroxyacetone phosphate toward glycerol-3-phosphate, reducing gluconeogenesis from glycerol. Thus, the redox imbalance induced by alcohol consumption leads to a large increase in NADH in the cytoplasm, inhibiting the flux of all major substrates into gluconeogenesis. The low blood glucose in blood (lacticacidemia). It also shifts cytosolic oxaloacetate toward malate, reducing gluconeogenesis from citric acid cycle intermediates, and shifts dihydroxyacetone phosphate toward glycerol-3-phosphate, reducing gluconeogenesis from glycerol. Thus, the redox imbalance induced by alcohol consumption leads to a large increase in NADH in the cytoplasm, inhibiting the flux of all major substrates into gluconeogenesis. The low blood glucose leads to a stress response (rapid heart beat, clammy skin), an effort to enhance stimulation of gluconeogenesis by combined action of glucagon and epinephrine leads to a stress response (rapid heart beat, clammy skin), an effort to enhance stimulation of gluconeogenesis by combined action of glucagon and epinephrine . The rapid breathing is a physiologic response to metabolic acidosis, resulting from the excess of lactic acid . The rapid breathing is a physiologic response to metabolic acidosis, resulting from the excess of lactic acid . . |
| LARGE CHILD BORN OF A DIABETIC MOTHER |
| A baby boy, born of a poorly controlled, chronically hyperglycemic, diabetic mother, was large and chubby (macrosomic) at birth (5 kg), but appeared otherwise normal. He declined rapidly, however, and within 1 h showed all of the symptoms of hypoglycemia, similar to the case of the baby girl born of a malnourished mother (p. 164). The difference, in this case, was that the boy was obviously on the heavy side, rather than thin and malnourished. |
Comment. This child has experienced a chronically hyperglycemic environment during uterine development. He adapted by increasing endogenous insulin production, which has a growth hormone-like activity, resulting in macrosomia. At birth, when placental delivery of glucose ceases, he has a normal blood glucose ceases, he has a normal blood glucose concentration and a substantial supply of hepatic glycogen. However, chronic hyperinsulinemia prior to birth probably represses gluconeogenic enzymes, and his high blood-insulin concentration at birth promotes glucose concentration and a substantial supply of hepatic glycogen. However, chronic hyperinsulinemia prior to birth probably represses gluconeogenic enzymes, and his high blood-insulin concentration at birth promotes glucose uptake into muscle and adipose tissue. The resultant insulin-induced hypoglycemia leads to a stress response, which was corrected by glucose uptake into muscle and adipose tissue. The resultant insulin-induced hypoglycemia leads to a stress response, which was corrected by glucose infusion. After 1-2 days, his ample body mass will provide a good reservoir for synthesis of blood glucose infusion. After 1-2 days, his ample body mass will provide a good reservoir for synthesis of blood glucose from muscle protein by gluconeogenesis. from muscle protein by gluconeogenesis. |
Gluconeogenesis requires both a source of energy and a source of carbons for formation of the backbone of the glucose molecule. The energy is provided by metabolism of
fatty acids released from adipose tissue. The carbon skeletons are provided from three primary sources: molecule. The energy is provided by metabolism of
fatty acids released from adipose tissue. The carbon skeletons are provided from three primary sources:
- lactate produced in tissues such as the red cell by anaerobic glycolysis;
- amino acids
 derived from muscle protein; derived from muscle protein; - glycerol released from triglycerides during lipolysis in adipose tissue.
|
Among these, muscle protein is the major precursor of blood glucose - the rate of gluconeogenesis is often limited by the availability of substrate, including the rate of proteolysis in muscle or, in some cases, muscle mass. During prolonged fasting, malnutrition or starvation, we lose both adipose and muscle mass. The fat is used both for the general energy needs of the body and to support gluconeogenesis, while most of the amino acids - the rate of gluconeogenesis is often limited by the availability of substrate, including the rate of proteolysis in muscle or, in some cases, muscle mass. During prolonged fasting, malnutrition or starvation, we lose both adipose and muscle mass. The fat is used both for the general energy needs of the body and to support gluconeogenesis, while most of the amino acids in protein are converted into glucose in protein are converted into glucose . .
|
|