| Metabolism of the carbon skeleton and the amino group are coordinated
|
Before the carbon skeletons of most amino acids can be metabolized, the α-amino group must be removed. The principal mechanism for removal of amino groups from the common amino acids can be metabolized, the α-amino group must be removed. The principal mechanism for removal of amino groups from the common amino acids is via transamination (above), or the transferring the amino group from the amino acid to a suitable α-keto acid acceptor, most commonly to α-ketoglutarate or oxaloacetate. Several enzymes, called amino transferases (or transaminases), are capable of removing the amino group from most amino acids is via transamination (above), or the transferring the amino group from the amino acid to a suitable α-keto acid acceptor, most commonly to α-ketoglutarate or oxaloacetate. Several enzymes, called amino transferases (or transaminases), are capable of removing the amino group from most amino acids and producing the corresponding α-keto acid. Aminotransferase enzymes use pyridoxal phosphate, a cofactor derived from the vitamin B6 (pyridoxine), as a key component in their catalytic mechanism. These structures and the net reaction catalyzed by amino transferases are shown in Figure 18.2. and producing the corresponding α-keto acid. Aminotransferase enzymes use pyridoxal phosphate, a cofactor derived from the vitamin B6 (pyridoxine), as a key component in their catalytic mechanism. These structures and the net reaction catalyzed by amino transferases are shown in Figure 18.2.
|
Nitrogen atoms are incorporated into urea from two sources from two sources
|
| Figure 18.2 The catalytic role of pyridoxal phosphate. Amino transferases or transaminases use pyridoxal phosphate as a cofactor and involve a pyridoxamine adduct which acts as an intermediate in transfer of an amino group between an α-amino acid and an α-keto acid. (A) Structures of the components involved. The cofactor, pyridoxal phosphate, is used in a variety of enzyme-catalyzed reactions involving both amino and keto compounds, including transamination and decarboxylation reactions. (B) Transamination involves both a donor α-amino acid (R1), and an acceptor α-keto acid (R2). The products are an α-keto acid derived from the carbon skeleton of R1 and an α-amino acid from the carbon skeleton of R2. |
| page 247 |  | | page 248 |
Figure 18.3 Sources of nitrogen atoms for the urea cycle. Nitrogen enters the urea cycle. Nitrogen enters the urea cycle from most amino acids cycle from most amino acids via transfer of the α-amino group to either α-ketoglutarate or oxaloacetate, to form aspartate or glutamate, respectively. Glutamate releases ammonia in the liver through the action of GDH. The ammonia is incorporated into carbamoyl phosphate, and the aspartate combines with citrulline to provide the second nitrogen for urea via transfer of the α-amino group to either α-ketoglutarate or oxaloacetate, to form aspartate or glutamate, respectively. Glutamate releases ammonia in the liver through the action of GDH. The ammonia is incorporated into carbamoyl phosphate, and the aspartate combines with citrulline to provide the second nitrogen for urea synthesis. Oxaloacetate and α-ketoglutarate can be repeatedly recycled to channel nitrogen into this pathway. synthesis. Oxaloacetate and α-ketoglutarate can be repeatedly recycled to channel nitrogen into this pathway. |
MEASUREMENT OF BLOOD UREA NITROGEN NITROGEN |
Blood urea measurements (BUN or blood urea measurements (BUN or blood urea nitrogen) are critical in monitoring patients with a variety of metabolic diseases in which the metabolism of amino acids nitrogen) are critical in monitoring patients with a variety of metabolic diseases in which the metabolism of amino acids may be affected, and in tracking the condition of individuals with renal disease. The traditional methodology used for measuring blood urea may be affected, and in tracking the condition of individuals with renal disease. The traditional methodology used for measuring blood urea levels has relied on the action of the enzyme urease which converts urea levels has relied on the action of the enzyme urease which converts urea to CO2 and ammonia. The resulting ammonia can be detected spectrophotometrically by formation of a colored compound on reaction with phenol or a related compound (the Berthelot reaction). Recently, the direct detection of urea to CO2 and ammonia. The resulting ammonia can be detected spectrophotometrically by formation of a colored compound on reaction with phenol or a related compound (the Berthelot reaction). Recently, the direct detection of urea by near infrared spectroscopy and fluorescence coupled assays has been proposed to decrease sample processing and increase sensitivity. by near infrared spectroscopy and fluorescence coupled assays has been proposed to decrease sample processing and increase sensitivity. |
 |
The transfer of an amino group from one keto acid carbon skeleton to another may seem to be unproductive and not useful in itself; however, when one considers the nature of the primary keto acid acceptors that participate in these reactions (α-ketoglutarate and oxaloacetate) and their products (glutamate and aspartate), the logic of this metabolism becomes clear. Nitrogen atoms are incorporated into urea exclusively from these two sources (Fig. 18.3), which link amino acid catabolism to energy metabolism. Ammonia produced primarily from glutamate (via the glutamate dehydrogenase (GDH) reaction, see Fig. 18.4) enters the urea exclusively from these two sources (Fig. 18.3), which link amino acid catabolism to energy metabolism. Ammonia produced primarily from glutamate (via the glutamate dehydrogenase (GDH) reaction, see Fig. 18.4) enters the urea cycle as carbamoyl phosphate. The second nitrogen is contributed to urea cycle as carbamoyl phosphate. The second nitrogen is contributed to urea by aspartic acid. Fumarate is formed in this process and may be recycled via the TCA cycle to oxaloacetate, which can
accept another amino group to reform aspartate or participate in either the TCA cycle or gluconeogenesis (see Fig. 18.7 and Chapter 12). Thus the funneling of amino groups from other amino acids by aspartic acid. Fumarate is formed in this process and may be recycled via the TCA cycle to oxaloacetate, which can
accept another amino group to reform aspartate or participate in either the TCA cycle or gluconeogenesis (see Fig. 18.7 and Chapter 12). Thus the funneling of amino groups from other amino acids into glutamate and aspartate provides the nitrogen for urea into glutamate and aspartate provides the nitrogen for urea synthesis in a form appropriate for the urea synthesis in a form appropriate for the urea cycle (see below). The other pathways that lead to the release of amino groups from some amino acids cycle (see below). The other pathways that lead to the release of amino groups from some amino acids through the action of amino acid oxidases or a dehydratase mechanism (Fig. 18.5) make relatively minor contributions to the flow of amino groups from amino acids through the action of amino acid oxidases or a dehydratase mechanism (Fig. 18.5) make relatively minor contributions to the flow of amino groups from amino acids to urea to urea . .
|
| MONOSODIUM GLUTAMATE REACTION |
| A healthy 30-year-old woman experienced the sudden onset of headache, sweating, and nausea after eating at an oriental restaurant. She felt weak, and experienced some tingling and a sensation of warmth in her face and upper torso. The symptoms passed after about 30 minutes and she experienced no further problems. Upon visiting her doctor the next day, she learned that some individuals react to foods containing high levels of the food additive monosodium glutamate, the sodium salt of glutamic acid. |
| Comment. The flu-like symptoms that develop, previously described as 'Chinese Restaurant Syndrome', have been attributed to central nervous system (CNS) effects of glutamate or its derivative, the inhibitory neurotransmitter, γ-amino butyric acid (GABA). Interestingly, studies have shown that this phenomenon causes no permanent CNS damage and that, although bronchospasm may be triggered in individuals with severe asthma, the symptoms are generally brief and completely reversible. |
| Glutamine and alanine are key transporters of amino groups between muscle and the liver
|
| page 248 |  | | page 249 |
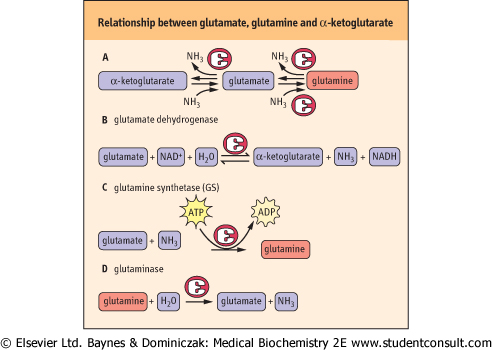
|
Figure 18.4 Relationship between glutamate, glutamine and α-ketoglutarate. The several forms of the carbon skeleton of glutamic acid have key roles in the metabolism of amino groups. (A) Three forms of the same carbon skeleton. (B) The GDH reaction is a reversible reaction that can produce glutamate from α-ketoglutarate or convert glutamate to α-ketoglutarate and ammonia. The latter reaction is important in the synthesis of urea because amino groups are fed to α-ketoglutarate via transamination from other amino acids because amino groups are fed to α-ketoglutarate via transamination from other amino acids . (C) Glutamine synthetase (GS) catalyzes an energy-requiring reaction with a key role in transport of amino groups from one tissue to another; it also provides a buffer against high concentrations of free ammonia in tissues. (D) The second half of the glutamine transport system for nitrogen is the enzyme, glutaminase, which hydrolyzes glutamine to glutamate and ammonia. This reaction is important in the kidney for management of proton transport and pH control. . (C) Glutamine synthetase (GS) catalyzes an energy-requiring reaction with a key role in transport of amino groups from one tissue to another; it also provides a buffer against high concentrations of free ammonia in tissues. (D) The second half of the glutamine transport system for nitrogen is the enzyme, glutaminase, which hydrolyzes glutamine to glutamate and ammonia. This reaction is important in the kidney for management of proton transport and pH control. |
In addition to the role of glutamate as a carrier of amino groups to GDH, glutamate serves as a precursor of glutamine, a process that consumes a molecule of ammonia. This is
important because glutamine, along with alanine (see Chapter 2), is a key transporter of amino groups between various tissues and the liver, and is present in greater concentrations than most other amino acids in blood. The three forms of the same carbon skeleton, α-ketoglutarate, glutamate, and glutamine, are interconverted via amino transferases, glutamate dehydrogenase, glutamine synthetase and glutaminase (Fig. 18.4). Thus glutamine can serve as a buffer for ammonia utilization, as a source of ammonia, and as a carrier of amino groups. Because ammonia is quite toxic, a balance must be maintained between its production and utilization. A summary of the sources and pathways that use or produce ammonia is shown in Figure 18.6. It should be noted that the glutamate dehydrogenase reaction is reversible under physiological conditions if amino groups are required for amino acid and other biosynthetic processes. in blood. The three forms of the same carbon skeleton, α-ketoglutarate, glutamate, and glutamine, are interconverted via amino transferases, glutamate dehydrogenase, glutamine synthetase and glutaminase (Fig. 18.4). Thus glutamine can serve as a buffer for ammonia utilization, as a source of ammonia, and as a carrier of amino groups. Because ammonia is quite toxic, a balance must be maintained between its production and utilization. A summary of the sources and pathways that use or produce ammonia is shown in Figure 18.6. It should be noted that the glutamate dehydrogenase reaction is reversible under physiological conditions if amino groups are required for amino acid and other biosynthetic processes.
|
Figure 18.5 Deamination of amino acids . The primary route for amino group removal is via transamination, but there are additional enzymes capable of removing the α-amino group. (A) l-amino acid oxidase produces ammonia and an α-keto acid directly, using flavin mononucleotide (FMN) as a cofactor. The reduced form of the flavin must be regenerated using molecular oxygen; this reaction is one of several that produce H2O2. The peroxide is decomposed by catalase. (B) A second means of deamination is possible only for hydroxyamino acids (serine and threonine), through a dehydratase mechanism; the Schiff base imine intermediate hydrolyzes to form the keto acid and ammonia. . The primary route for amino group removal is via transamination, but there are additional enzymes capable of removing the α-amino group. (A) l-amino acid oxidase produces ammonia and an α-keto acid directly, using flavin mononucleotide (FMN) as a cofactor. The reduced form of the flavin must be regenerated using molecular oxygen; this reaction is one of several that produce H2O2. The peroxide is decomposed by catalase. (B) A second means of deamination is possible only for hydroxyamino acids (serine and threonine), through a dehydratase mechanism; the Schiff base imine intermediate hydrolyzes to form the keto acid and ammonia. |
|